2
2
GENERAL INFORMATIONS
WARNINGS
• Please read this manual carefully before carrying out any operation on
TOP SPIN wheelchair.
• Before use the device verify the package conditions (see par. Packaging, transport,
delivery and stocking in this manual).
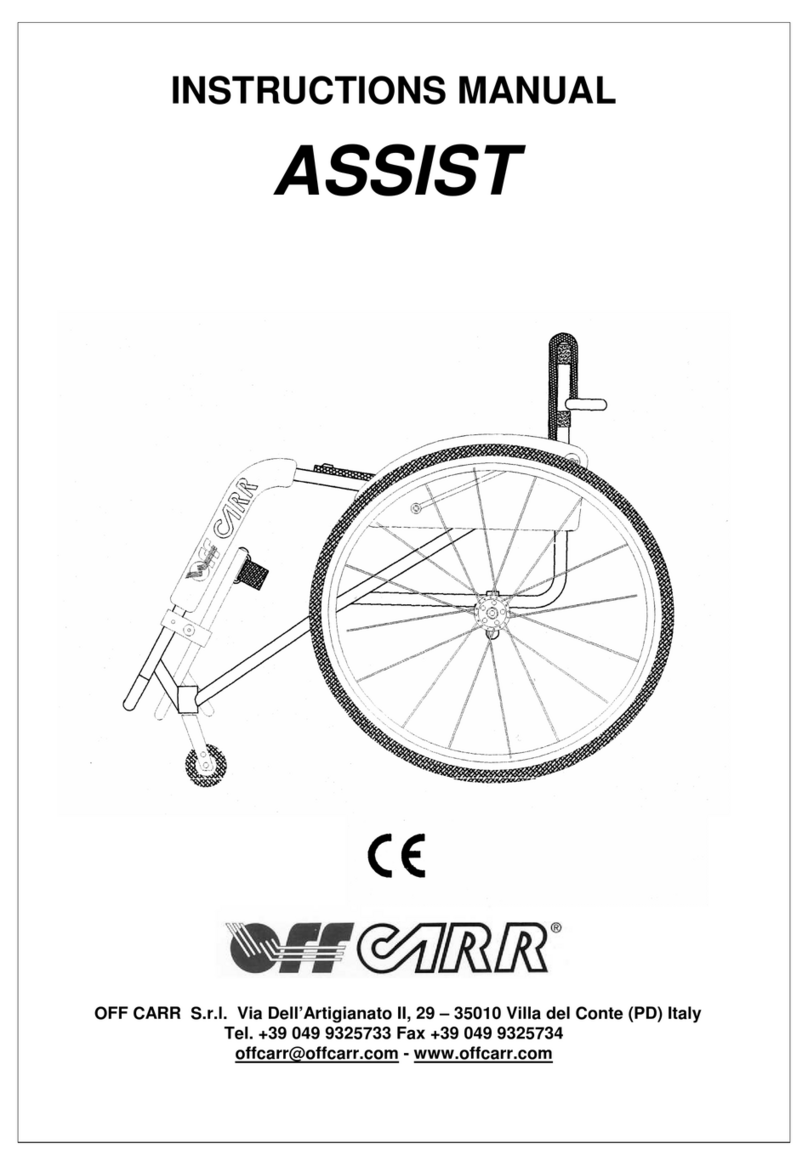
• Every wheelchair is labelled with an Identification Number (Matricola), the
manufacturer address and CE mark. This label cannot be removed for any reason to
maintain trace-ability and guarantee terms of the device.
• TOP SPIN models are designed and produced to compensate a physical handicap
following 93/42/EEC EN Directive Medical Device Essential Requirements.
According with above Directive, appendix IX, par. III. 1.1., TOP SPIN 1 or TOP SPIN
2 are classified as non-invasive medical device of class 1.
• The use of TOP SPIN or its components is forbidden for any purpose other than
those indicated in this manual.
• For any problems or fault or breakage, please notify immediately to your supplier
reporting the following indications:
a. Model
b. Identification number (“Matricola” printed on the frame gold label).
c. Fault description
Packaging, transport, delivery and stocking.
All wheelchairs are shipped in a closed cardboard cases to protect them from knocks
and dust.
The package includes the wheelchair TOP SPIN 1 or 2 configured as per your request,
and this Instruction manual.
The wheelchair must be transported in trucks that protect it from atmospheric agents, as
indicated on the packing case.
Upon receipt, check the case integrity and in case of problems notify them on the waybill.
Open the package, remove the wheelchair and check if it is not bent or scratched or
damaged. In case of problems note your remark on the waybill and notify them
immediately to the forwarder company.
Once these checks have been carried out, place again the wheelchair in its package
keeping it in a dry place until it will be used.
Do not place any objects over the packing case.