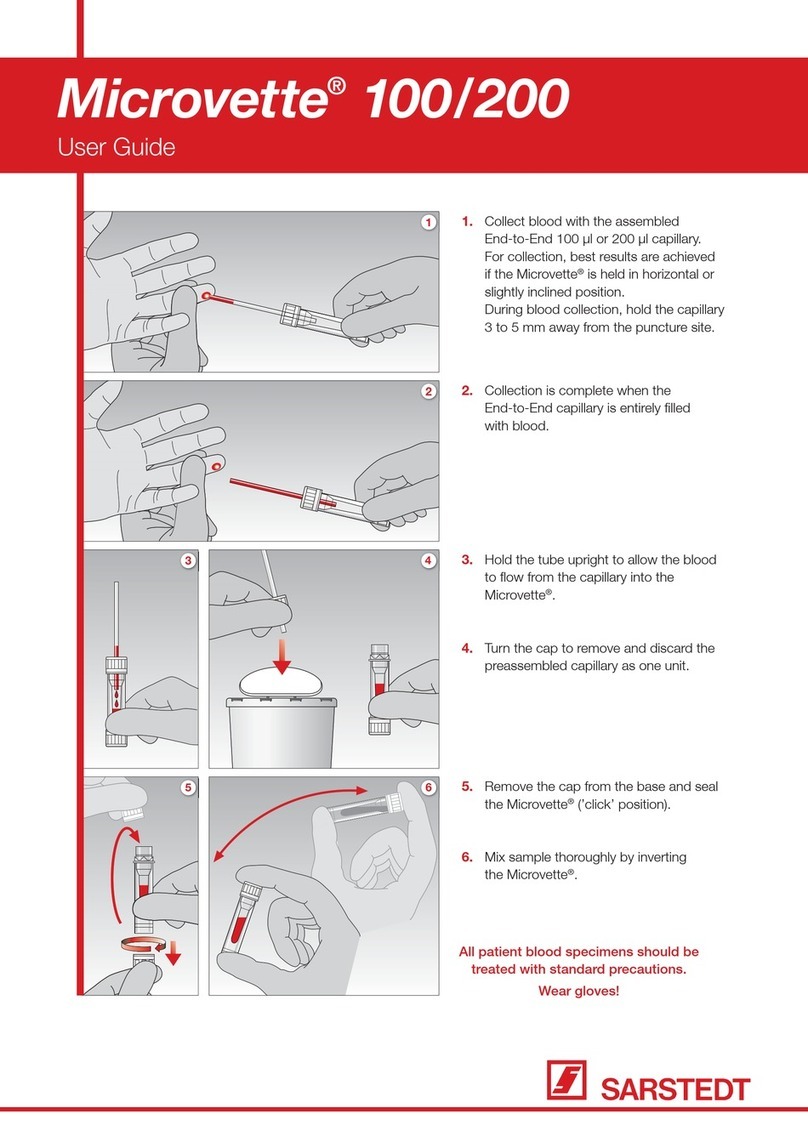
4
Instructions for use SAHARA-TSC
1 Safety information
• Please note the information in the service manual.
• The device may only be operated by trained medical personnel.
• The device may only be installed and operated in areas of professional health care facilities with no strong electromagnetic
interference elds. Portable RF communication equipment may aect the device functions and should therefore not be
used at a distance less than 30 cm from parts and cables of the device.
Operate the device only with the mains cable supplied. Using a mains cable other than the original cable supplied may
lead to a higher electromagnetic emission or reduced electromagnetic immunity of the device and result in a malfunction.
This device should not be operated directly beside or stacked with other devices, since this may lead to a malfunction.
If this is however necessary, the devices should be observed with respect to their correct operation.
• To prevent the risk of electric shock, the device must only be connected to a mains supply with a protective earth
connection.
• Check the device for visible signs of damage before switching on. If you notice any safety relevant damage the device
must not be used.
• If the device should be connected to an IT network, the integration of IT devices other than those specied in Chapter 16,
changes to the IT network conguration, additional connection or removal of IT devices, and a software update on the IT
devices used can lead to risks for patients, operators or third parties that were previously unknown. These risks should be
analysed and assessed by the operator.
• To remove leaked liquids do not tilt the device.
• To avoid possible crushing of ngers install and remove the agitation plate only when the device has been turned o.
• If the device has to be opened during cleaning or servicing, it must be turned o and disconnected from the local power
supply by unplugging the mains cable since some device parts are under voltage even when the device has been
turned o.
• The device must not be used within the patient environment.
• The leukapheresis product within the device must not be connected to the patient.
• During an on-going tempering process the leukapheresis product must not be removed from the device.
• Do not modify this equipment without authorization of the manufacturer.
• Any serious incident that has occurred in relation to the device should be reported to the manufacturer and the competent
national authority in which the user is established.
2 Explanation of symbols and instructions
Follow instructions for use
WARNING
Important information. If ignored a serious or life-threatening injury may occur.
WARNING
Important information. If ignored an electrical shock due to dangerous voltage may occur.
CAUTION
Important information. If ignored a minor injury may occur.
CAUTION
Helpful information on the appropriate use of the device. If ignored an operating error,
malfunction or device defect may occur
Permissible pressure range