Indications
Synthes Spine 3
The Synthes USS (including the Click’X, USS VAS variable axis
components, and Pangea), Click’X Monoaxial, Pangea
Monoaxial, Dual-Opening and the Small Stature USS (which
includes small stature and pediatric patients) are noncervical
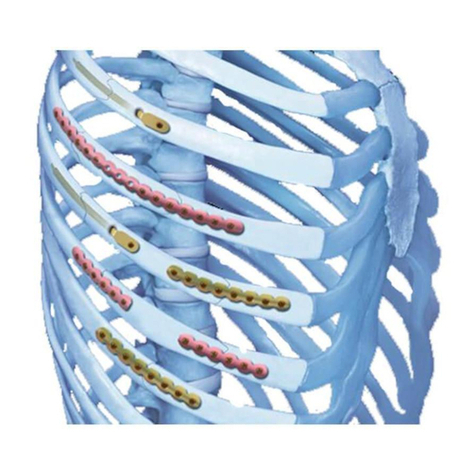
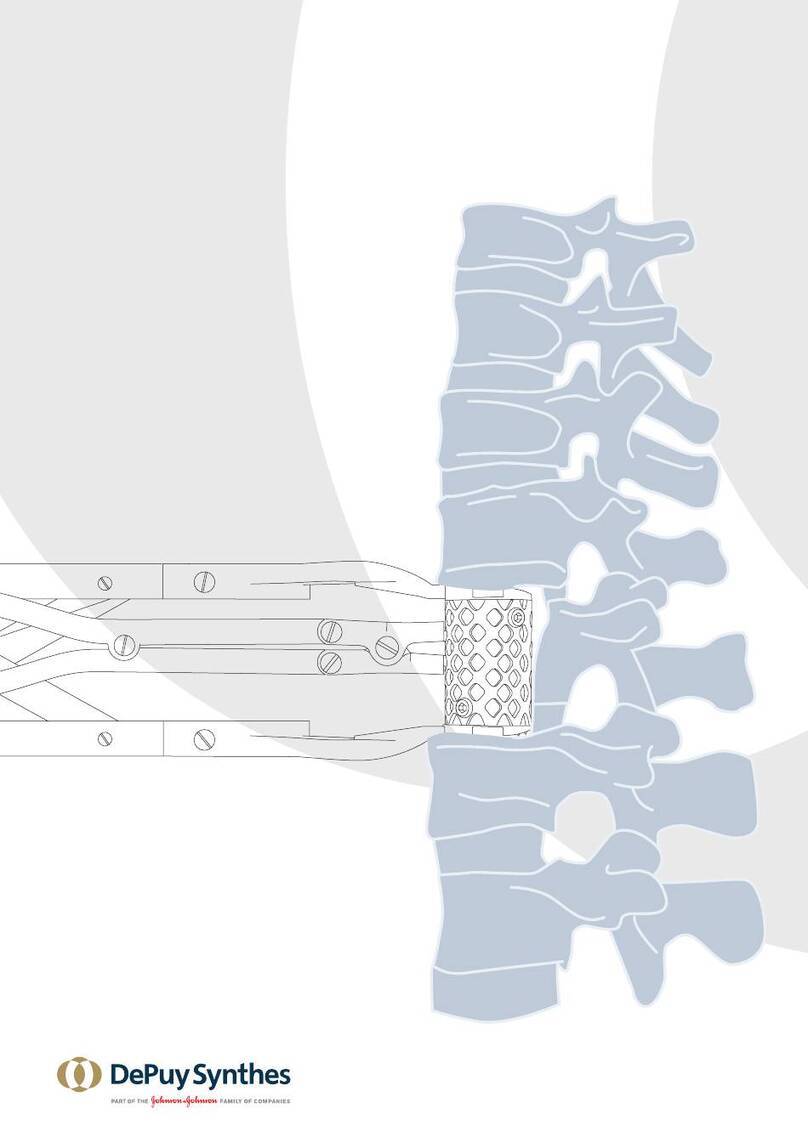
spinal fixation devices intended for use as posterior pedicle
screw fixation systems (T1–S2), aposterior hook fixation
system (T1–L5), or as an anterolateral fixation system (T8–L5).
Pedicle screw fixation is limited to skeletally mature patients
with the exception of the Small Stature USS. These devices
are indicated for all of the following indications regardless of
the intended use: degenerative disc disease (defined as disco-
genic back pain with degeneration of the disc confirmed by
history and radiographic studies), spondylolisthesis, trauma
(i.e., fracture or dislocation), deformities or curvatures (i.e.,
scoliosis, kyphosis, and/or lordosis, Scheuermann’s disease),
tumor, stenosis, pseudoarthrosis, and failed previous fusion.
When treating patients with degenerative disc disease (DDD),
transverse bars are not cleared for use as part of the posterior
pedicle screw construct.
When used with the 3.5 mm/6.0 mm parallel connectors,
the Synthes USS (including the Click'X, USS VAS variable axis
components, and Pangea), Click'X Monoaxial, Pange Mono-
axial, and Dual-Opening USS can be linked to the CerviFix
system. In addition, when used with 3.5 mm/5.0 mm parallel
connectors, the Synthes Small Stature USS, can be linked to
the CerviFix system. When used with the 5.0 mm /6.0 mm
parallel connectors, the Synthes Small Stature USS can be
linked to the Synthes USS (including the Click'X, USS VAS
variable axis components, and Pangea), the Click'X Monoax-
ial, PangeaMonoaxial, and Dual-Opening USS systems.
In addition, Synthes USS (including the Click’X, USS VAS
variable axis components, and Pangea), Click’X Monoaxial,
PangeaMonoaxial and the Dual-Opening USS can be inter-
changed with all USS 6.0 mm rods and transconnectors.