Warning And Caution
Safety:
1.Warning, Do not modify this equipment without authorization of manufacturer.
2.Warning, Keep away from the wet medical equipment such as drip or other similar liquid simulation as
far as possible.
3.Warning, Check the sensor site every half an hour to ensure adequate blood circulation, intact skin,
and appropriate sensor location. Otherwise, it may cause skin damage, compressive necrosis, or
inaccurate measurement readings.
4. Warning, With the increasing number of radio devices or other noise sources from electric
equipment in health care departments , our product may be interrupted when working because of their
interference. The closer the distance between each other is or stronger the signal is, the more serious
the interference will be.
The electromagnetic interference sources in health care departments may include:
(1).Electronic surgical instruments communications equipment (2).Mobile Phones
(3).Automotive two-way wireless communications equipment (4).Electronic apparatus
(5).High-definition television
In this interference, the measurement values may deviate, or the device may not work. When
interfered, the product may produce abnormal phenomenon: unstable reading values, outages or other
functions of error. If such a case, the use of the site should be checked to identify interference and the
elimination of the following measures:
(1)Shut down the equipment in the vicinity and then re-open in order to identify interference equipment;
(2)To change the direction or location of the interference equipment;
(3)To increase the distance between the product and interference sources.
5.Warning,Do not put the battery close to the fire or into the fire to avoid the battery explosion. Do not
use the battery when it leaks or molds.
6.Warning, Device conforms to the requirement of RoHS directive.
7.Warning, Device application component materials are certified for biological compatibility.
8. Warning, Do not leave the oximeter unattended around children or infants. Small items such as the
battery door, battery,and lanyard may become choking hazards if swallowed. Infants or children may
be entangled in the lanyard, thus causing strangulation.
9.Warning, Do not stare at the light (the infrared is invisible) which emitted from the oximeter,which is
harmful to the eyes.
10.Warning, Do not use the oximeter for purposes other than its intended use. Do not place the
oximeter on edema or fragile tissues.
11.Warning, Do not use the oximeter close to a permanent magnet.
12.Warning, Do not use the finger pulse oximeter in an MRI or CT environment.
13.Warning, Do not use the finger pulse oximeter during defibrillation and electrosurgey.
14.Warning, Do not use the finger pulse oximeter in the presence of flammable anesthetics or other
flammable substances, oxygen-enriched environments, or nitrous oxide to avoid the risk of explosion.
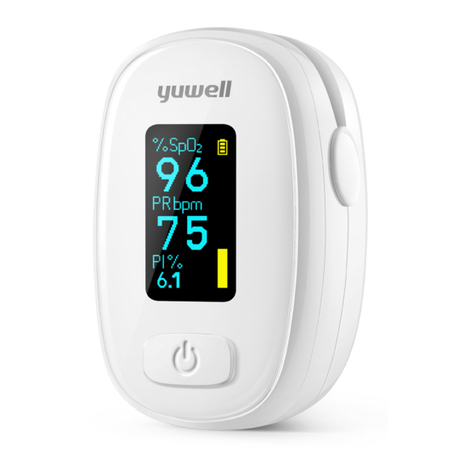
15.Caution,When using this product, you only need to fix it on your fingers. Excessive pressure on your
fingers may cause skin damage.
16.Caution, The maximum skin surface temperature is below 41℃(106℉) when measured in a 35℃
(95℉)environment, which has been verified by measuring the skin surface temperature via a Finger
Pulse Oximeter under the reasonable worst conditions.
17.Caution, Please pay attention to product storage to prevent damage caused by pets,pests or
children.
Performance:
18.Warning, Do not self-diagnose or self-medicate based on measurement results.It must be used in
conjunction with other methods of assessing clinical signs and symptoms.
19.Warning, Do not use the the finger pulse oximeter in situations where alarms are required. The
device has no Alarm System.
20.Warning, The effect of sensor and electrode degradation or electrode loosening may reduce the
performance of the measurement or cause other problems.
21. Warning,The thickness of the little finger may be smaller than the minimum size range for which this
product is designed, so we recommend using index finger, middle finger and ring finger instead of small
finger.
22. Warning, Do not use the oximeter if it shows signs of damage or is suspected of being damaged.
Inaccurate or no readings may result from internal damage.
23. Warning, Avoid the following to reduce the risk of SpO2 inaccuracy:
(1)Improper placement of the oximeter.
(2)Significant levels of dysfunctional hemoglobin (such as carbonyl hemoglobin or methemoglobin);
(3)Blood vessels contain dyes (such as indigo green or methylene blue).
(4)External paints and substances (e.g. nail polish, nail polish, glitter, etc.).
(5)The user is wearing a high-pressure cuff when measuring.
(6)Avoid placing the oximeter on any arm with an arterial cannula or blood pressure cuff.
(7)Elevated bilirubin levels. Severe anemia. (8)Venous congestion. Venous pulsation.
(9)Arterial perfusion levels are extremely low. (10)Excessive physical activity.
(11)During cardiac arrhythmia.
24.Warning, Keep the oximeter away from electrical equipment that emits radio frequencies to minimize
radio interference. RF may result in inaccurate or inaccurate readings.
25.Warning, Properly apply and avoid using the oximeter in strong ambient light sources, fluorescent
lights, infrared heat lamps, and direct sunlight to minimize interference that may result in unreadable or
inaccurate readings.
26.Caution, The display may not read clearly when exposed to direct sunlight or bright light.
27.Caution,Do not use a functional tester to evaluate the accuracy of the Finger Pulse Oximeter. The
functional tester shall only be used to check whether a unit is working properly.
28.Caution,SpO2 is empirically calibrated in healthy adult volunteers with normal levels of carboxyhe-
moglobin(COHb) and methemoglobin(MetHb).
29.Caution, Please replace the battery when a low battery remind appears.
Maintenance and others:
30. Warning, Please do not repair and maintain the equipment during use.
1 2