24
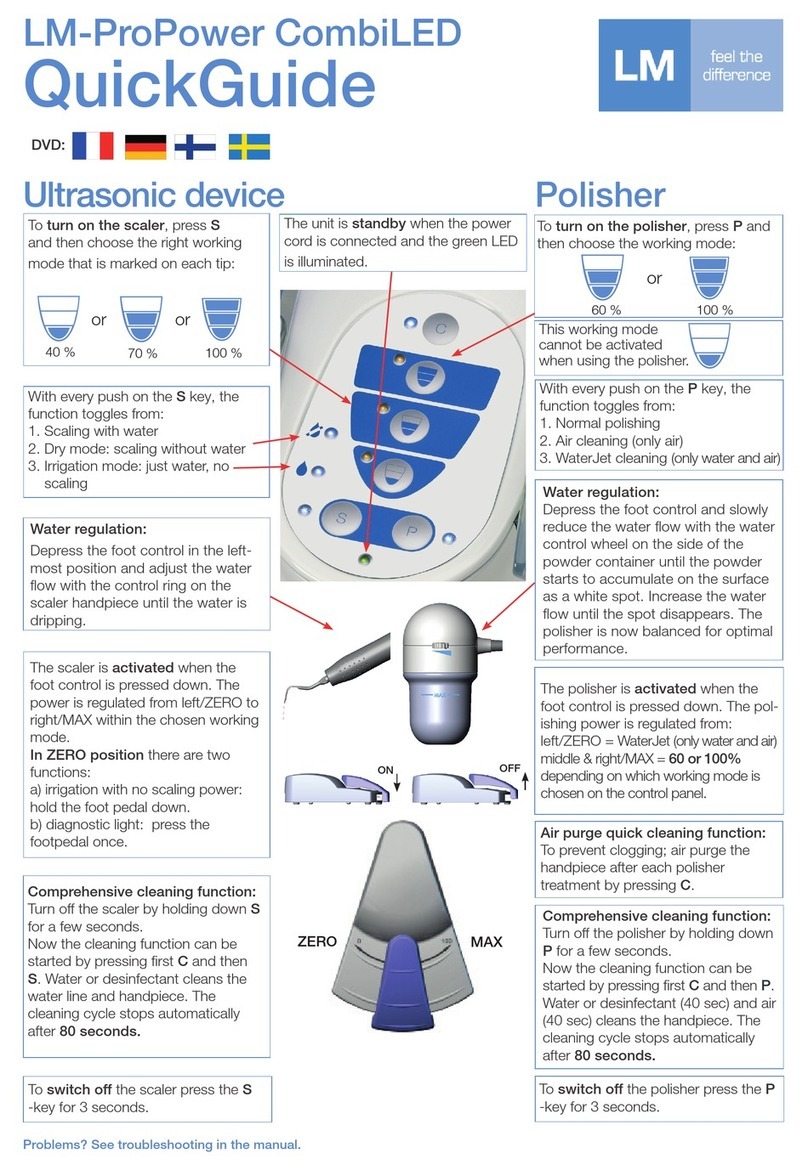
We stronglyadvise the use of a class B autoclave.
All other methods of sterilisation must be avoided.
Read the instructions for use provided by the autoclave manufac-
turer.
Maintain the space between the bags and do not overload the
autoclave.
Only sterilise instruments that have been predisinfected, cleaned,
lubricated and tested.
Remove the instruments from the MD Impactor before sterilisa-
tion (See Section 5.5).
Make sure that the MD (Impactor + Instruments) do not have any
areas of corrosion or cracks, and checkthat it is working properly.
Ensure that the MD (Impactor + Instruments) are dry; if necessary,
dry any residual waterusing medical quality pressurised air.
Use the sterilisation bags that are designed for the MD (Impactor+
Instruments) and for the autoclave, or use a sterilisation cassette.
Each bag should always only contain one MD or sterilisation tray.
In order to avoid any retention of water, place the bag in the
autoclave in such a way that any hollow parts are face
down.
If the pre-vacuum autoclave offers a number of different cycles,
choose a cycle for MD (at least 135°C at 2.13 bar (275°F at 30.88
psi) for 3 minutes with a drying timeof 16 minutes).
After every sterilisation cycle, check that there is no water re-
maining on the inside and outside of the packaging.
Check that the change in colour of the flow indicator is correct.
7.6. Storage:
Keep the MD (Impactor + Instruments) in the sterilisation
bags away from light, moisture and contamination of any
kind. Follow the recommendations of the packaging manufacturer.
The duration for which the MD (Impactor + Instruments) may be
kept after sterilisation may not exceed 1 month.
Label the MD (Impactor + Instruments), specifying the expiration
date. After the expiration date, repeat the cleaning and sterilisa-
tion cycle.
VIII. REPAIRS
In the case of device failure, please contact your authorised
distributor, or contact our after-sales service department directly.
Repairs may only be carriedout by an authorised repair service or
by the Anthogyr after-sales service department, and only using
original Anthogyr replacement parts.
For any servicing or repair work, the MD must be returned com-
plete and sterile with proof of sterility. It must be accompanied by
a document that describes the problem in question and also
includes the complete contact details of the practitioner who used
the device.
Exchange of replacement parts is possible for 7 years after sales
discontinuation.
CONTACT DETAILS: AFTER-SALES SERVICE
AFTER-SALES SERVICE DEPARTMENT
Anthogyr
2237 Avenue André Lasquin -74700 Sallanches - FRANCE
Direct line: +33 (0)4 50 58 50 53
Mail: sav@anthogyr.com
IX. GUARANTEE
These MD (Impactor + Instruments) are guaranteed for parts and
labour against any defect in manufacture for a period of 12
months from the date of invoicing. This guarantee does not apply
to wear parts and does not cover transport costs. For claims to be
considered under the guarantee, please enclose a copy of the
invoice or the delivery note with the MD (Impactor + Instruments) .
Any modification of or addition to the product without the express
consent of Anthogyr will nullify this guarantee.
The guarantee shall become null and void if the technical instruc-
tions supplied with all of our equipment are not observed.
Anthogyr cannot be held responsible for damage and ensuing
damage resulting or possibly resulting from normal wear and
tear, incorrect use, cleaning or maintenance, non-compliance
with the provisions regarding use or connection, scaling or corro-
sion, impurities in the water supply system or abnormal chemical
or electrical effects, or non-compliance with the instructions for
use, maintenance or installation provided by Anthogyr, and other
manufacturer instructions.
E
D
Français
English (UK)
Deutsch
Español
Italiano
Português
Nederlands
中国的
25
English (US)
X. ACCESSORIES - INSTRUMENTS
Instruments
Accessories
XI. CONDITIONS OF STORAGE & TRANSPORT
XII. DISPOSAL OF THE PRODUCT
The MD (Impactor + Instruments) must be sterilised before dispos-
al in order to prevent the risk of third-party contamination.
On the basis of current knowledge, the product does not contain
any substances that are harmful to the environment. Observe
national legislation, standards and provisions with regard to
disposal.
References Designation
OSTSCC34 OsteotomeØ3.4 concavestraight
OSTEOSAFE Solution
OSTSCC40 OsteotomeØ4.0 concavestraight
OSTSCC46 OsteotomeØ4.6 concavestraight
OSTSCC52 OsteotomeØ5.2 concavestraight
OSTSCX34 OsteotomeØ5.2 convex straight
OSTSCX40 OsteotomeØ4.6 convex straight
OSTSCX46 OsteotomeØ4.0 convex straight
OSTSCX52 OsteotomeØ3.4 convex straight
OSTECC34 Osteotome Ø3.4 concave bayonet
OSTECC40 Osteotome Ø4.0 concave bayonet
OSTECC46 Osteotome Ø4.6 concave bayonet
OSTECC52 Osteotome Ø5.2 concave bayonet
OSTECX34 Osteotome Ø3.4 convex bayonet
OSTECX40 Osteotome Ø4.0 convex bayonet
OSTECX46 Osteotome Ø4.6 convex bayonet
OSTECX52 Osteotome Ø5.2 convex bayonet
OPIP100 Tip for abutment 0°/7°
SAFELOCK®
Solution
OPIP200 Tip for abutment 15°/23°
OPIP400 Tip for extra-orally cemented prosthesis
PERIO01 Cx right Fine Periotome
EXO SAFE Solution
PERIO02 Cx rightWide Periotome
PERIO03 Cx Ang. Vertical Periotome
PERIO04 Cx Ang. Horizontal Periotome
PERIO05 Cc right Fine Periotome
PERIO06 Cc right Medium Periotome
References Designation
1930X Lubricating spray All
solutions
1932X ISO type E connection tip