Arjo Prioma Semi Electric Couch Series User manual
Other Arjo Medical Equipment manuals

Arjo
Arjo MAA2000-XS User manual

Arjo
Arjo Enterprise ENT-ACC06 User manual
Arjo
Arjo Huntleigh Hydroven 12 LymphAssist... User manual
Arjo
Arjo Carevo Operating instructions

Arjo
Arjo Typhoon User manual

Arjo
Arjo CARENDO Operating instructions
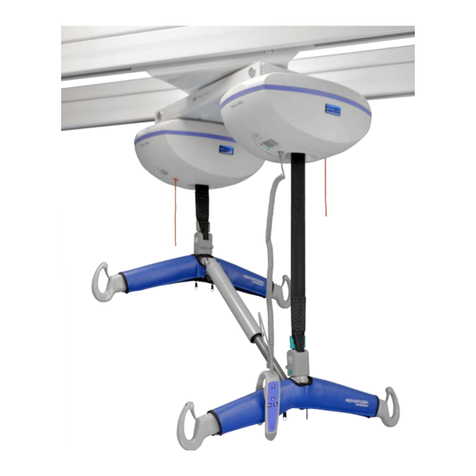
Arjo
Arjo Maxi Sky 2 PLUS User manual

Arjo
Arjo Enterprise 5000X User manual

Arjo
Arjo Walker Manual

Arjo
Arjo Prioma User manual
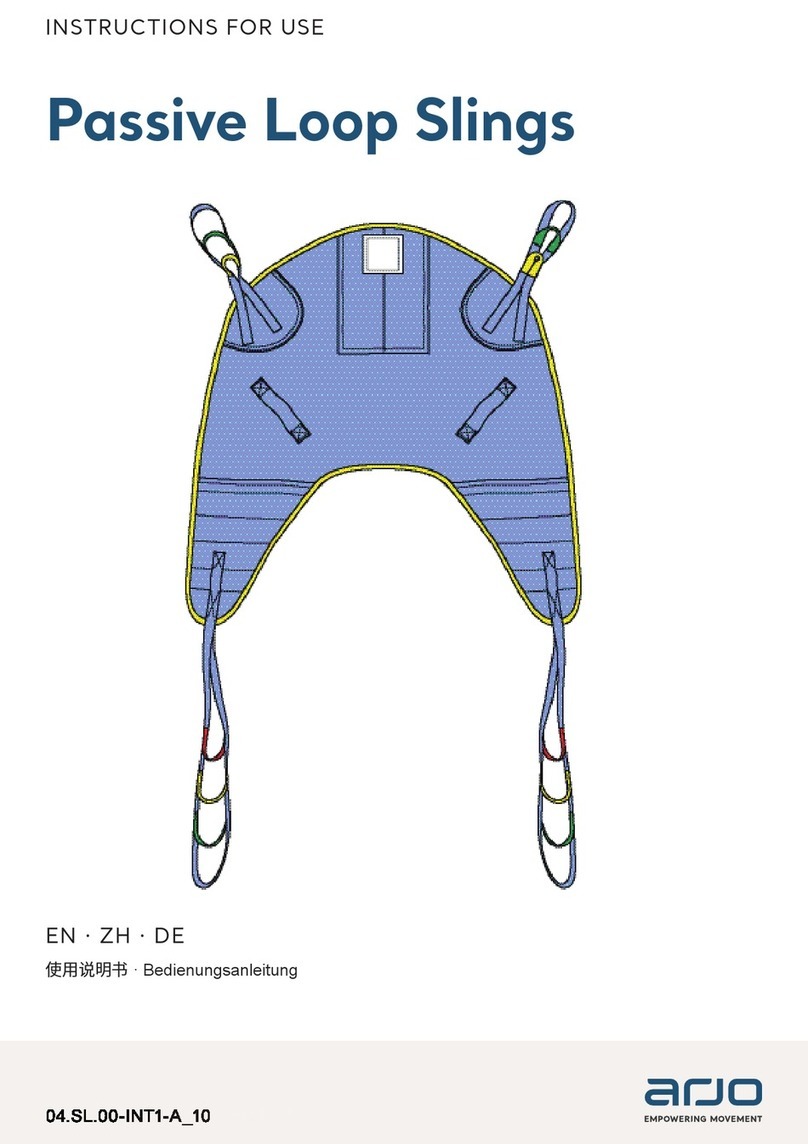
Arjo
Arjo MLAAS2000 User manual
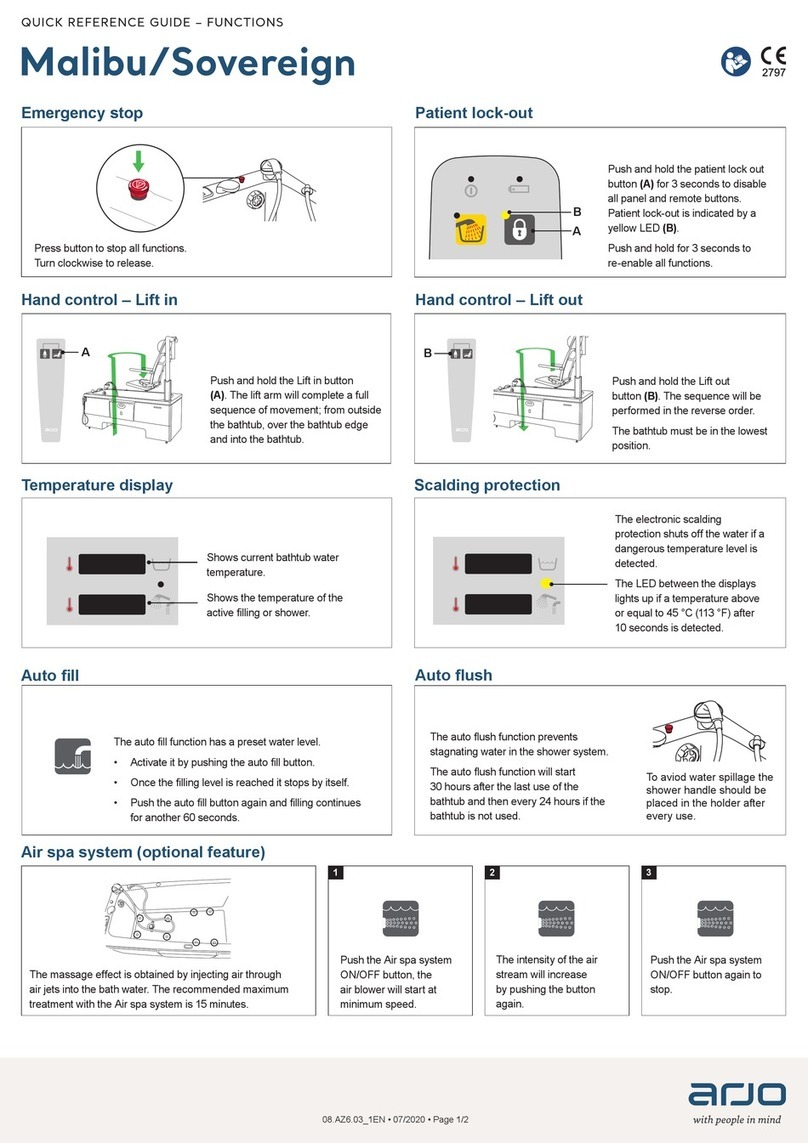
Arjo
Arjo Malibu User manual
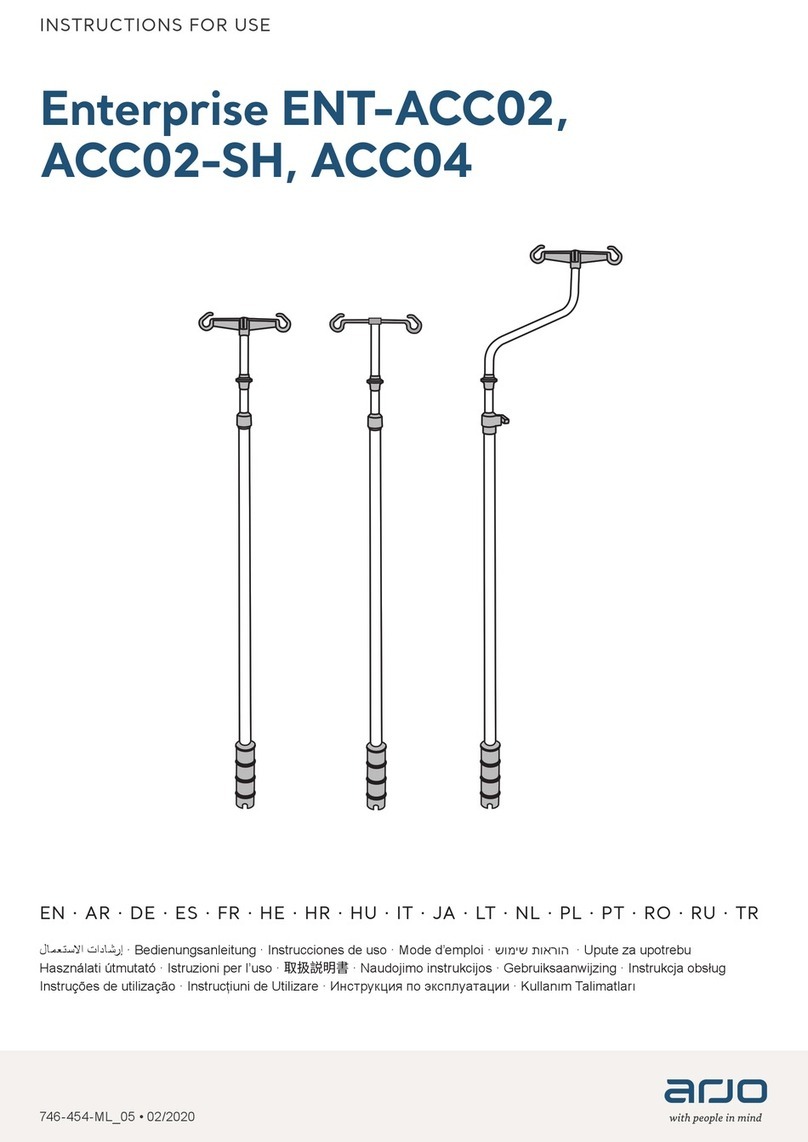
Arjo
Arjo Enterprise ENT-ACC02 User manual

Arjo
Arjo Maxi Sky 2 User manual
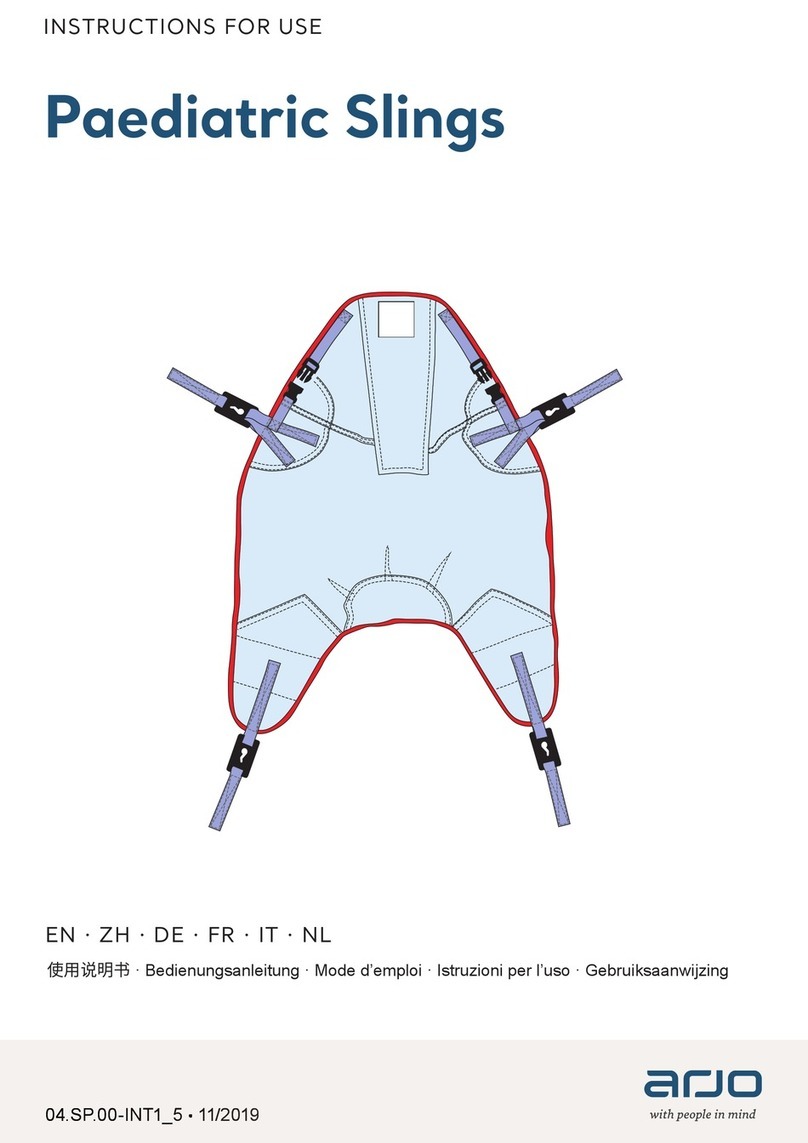
Arjo
Arjo MAA4020M User manual

Arjo
Arjo Enterprise 9000X User manual
Arjo
Arjo Citadel User manual
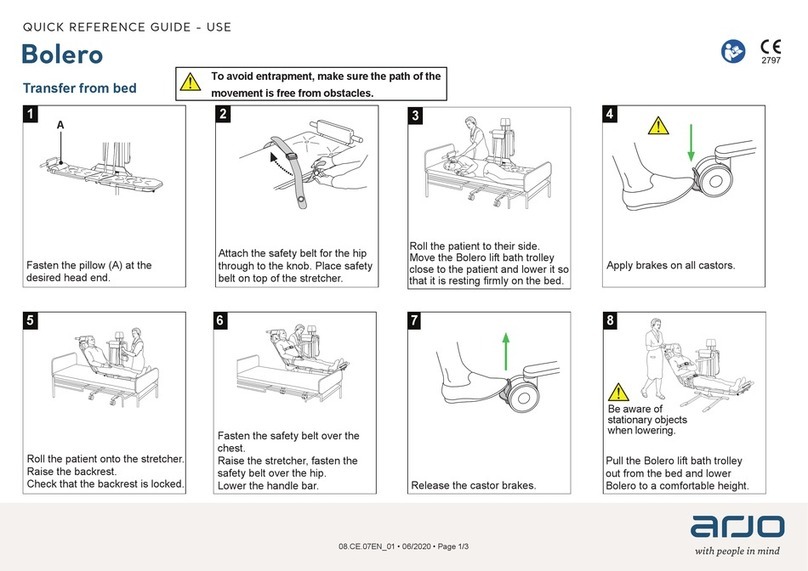
Arjo
Arjo Bolero User manual

Arjo
Arjo Maxi Sky 1000 User manual

Arjo
Arjo Tenor Installation guide