1.0 Introduction................................................................................................................................................. 4-7
1.1 Notes on operating instructions................................................................................................................................................ 4
1.2 Intended use................................................................................................................................................................................... 5
1.3 Function ........................................................................................................................................................................................... 7
1.4 Transport and storage ................................................................................................................................................................. 8
1.5 Explanation of symbols................................................................................................................................................................ 9
2.0 Safety advice............................................................................................................................................ 10-13
2.1 Notice ......................................................................................................................................................................................10-11
2.2 Caution...........................................................................................................................................................................................12
2.3 Warning..........................................................................................................................................................................................12
3.0 Setting up and starting up .....................................................................................................................14-18
3.1 Scope of delivery..........................................................................................................................................................................14
3.2 Device overview ...........................................................................................................................................................................15
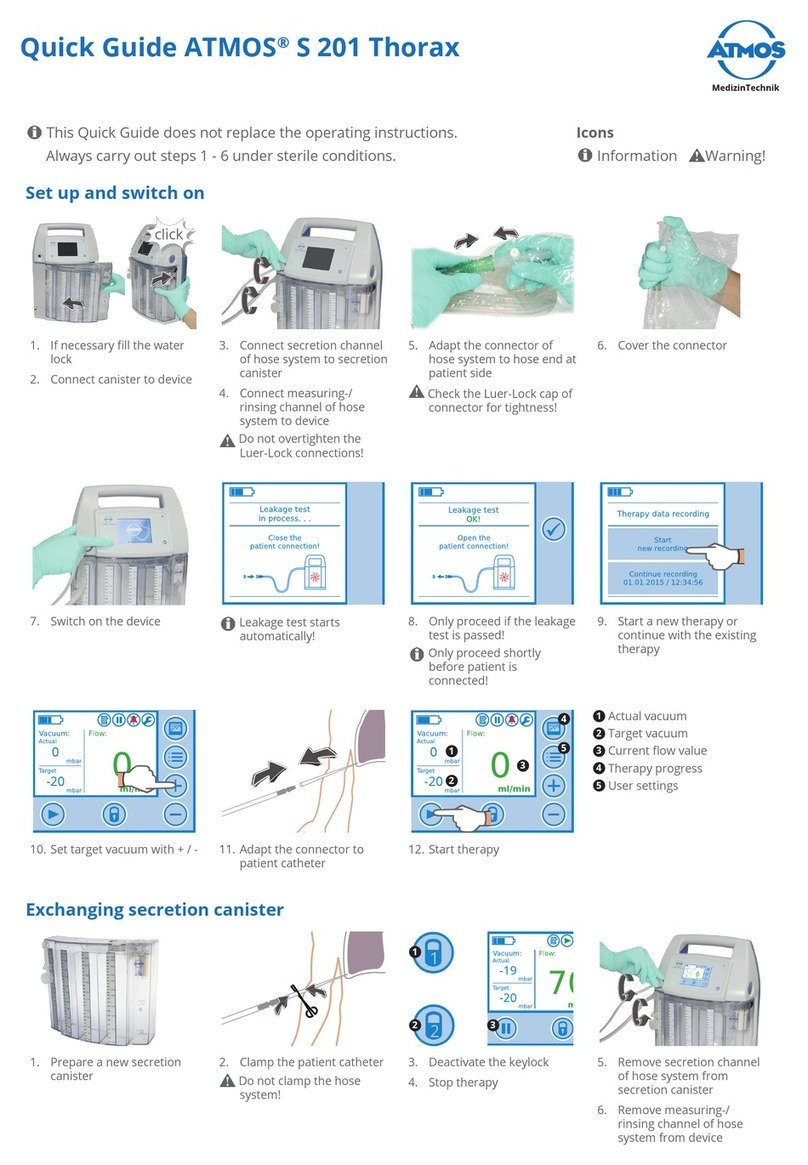
3.3 Starting up.....................................................................................................................................................................................16
3.3.1 Charging the battery................................................................................................................................................................16
3.3.2 Secretion canister ....................................................................................................................................................................17
3.3.2.1 Secretion canister overview ...............................................................................................................................................17
3.3.2.2 Pop-Off valve..........................................................................................................................................................................17
3.3.2.3 Connecting the secretion canister ...................................................................................................................................17
3.3.2.4 Exchanging the secretion canister ...................................................................................................................................18
3.3.3 Connecting the hose system.................................................................................................................................................18
4.0 Operating..................................................................................................................................................19-29
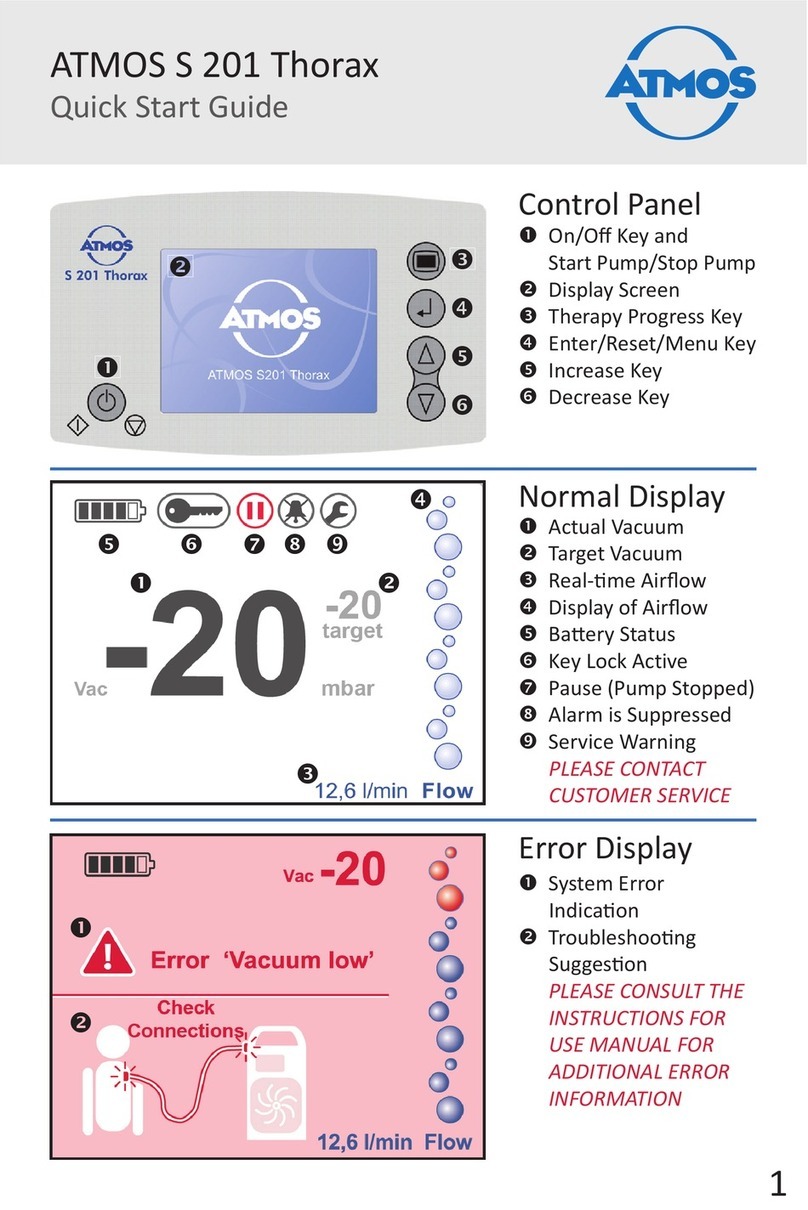
4.1 Explanation of the display .........................................................................................................................................................19
4.2 Buttons and display symbols..............................................................................................................................................20-21
4.3 Switch on .......................................................................................................................................................................................21
4.4 Leakage test..................................................................................................................................................................................22
4.5 Function .........................................................................................................................................................................................23
4.5.1 Target vacuum ...........................................................................................................................................................................23
4.5.2 Suction .........................................................................................................................................................................................23
4.6 Release of keylock .......................................................................................................................................................................24
4.7 Therapy progress.........................................................................................................................................................................25
4.7.1 Shorttime.....................................................................................................................................................................................25
4.7.2 Longtime......................................................................................................................................................................................25
4.7.3 Transfer of therapy data..........................................................................................................................................................26
4.7.4 Reading out the therapy data ................................................................................................................................................27
4.8 Switch off ........................................................................................................................................................................................28
4.9 User settings............................................................................................................................................................................28-29
5.0 Warning messages ..................................................................................................................................30-31
6.0 Functions....................................................................................................................................................... 31
6.1 Hose rinsing ...................................................................................................................................................................................31
7.0 Accessories, consumables and spare parts .........................................................................................32-34
7.1 Attachment of the universal bracket .......................................................................................................................................32
7.2 Attaching/removing the ATMOS®C 051 Thorax to/from the universal bracket ...........................................................33
7.3 Connecting of shoulder belt ......................................................................................................................................................34
8.0 Cleaning and care....................................................................................................................................35-37
8.1 General information on cleaning and disinfection..............................................................................................................35
8.2 Cleaning of the device surface .................................................................................................................................................36
8.3 Recommended disinfectants....................................................................................................................................................36
8.4 Hygiene plan .................................................................................................................................................................................37
9.0 Maintenance and Service....................................................................................................................... 38-39
9.1 Basic information.........................................................................................................................................................................38
9.2 Repairs ...........................................................................................................................................................................................38
9.3 Handling of rechargeable batteries .........................................................................................................................................39
10.0 Eliminating errors ...................................................................................................................................... 40
11.0 Technical data ............................................................................................................................................ 41
12.0 Disposal ....................................................................................................................................................... 43
13.0 Notes on EMC......................................................................................................................................... 44-47
Table of contents