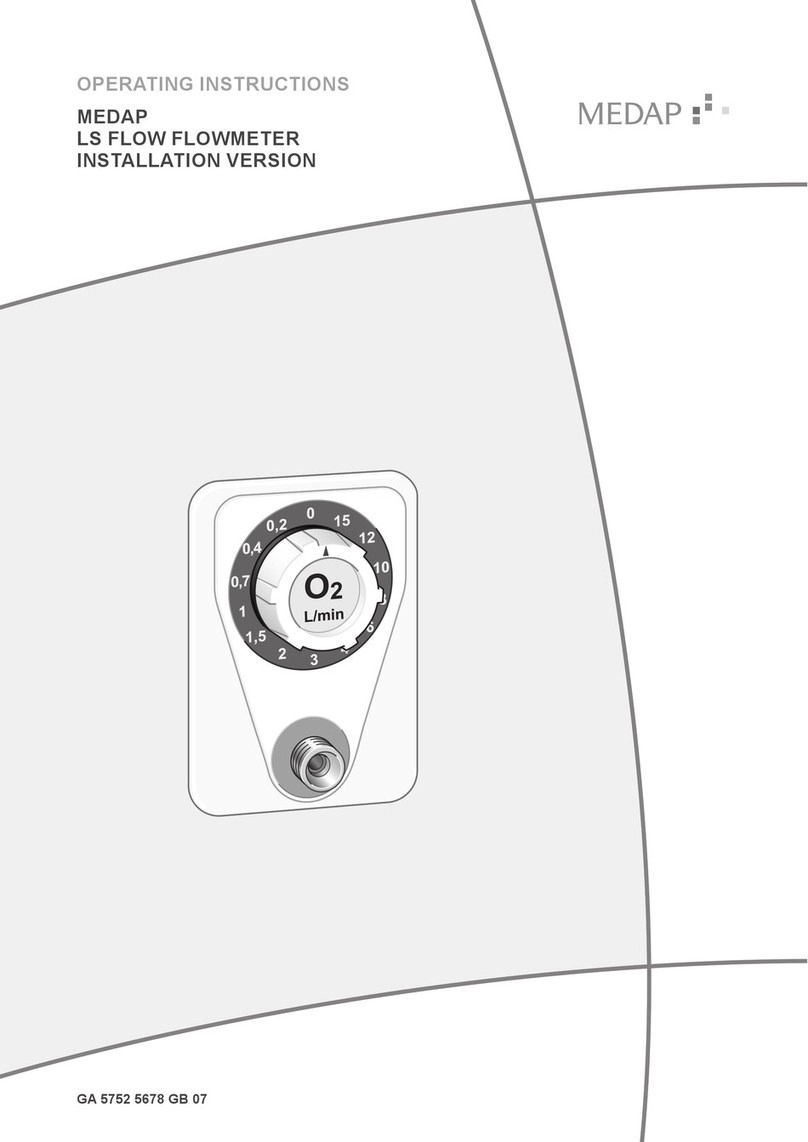
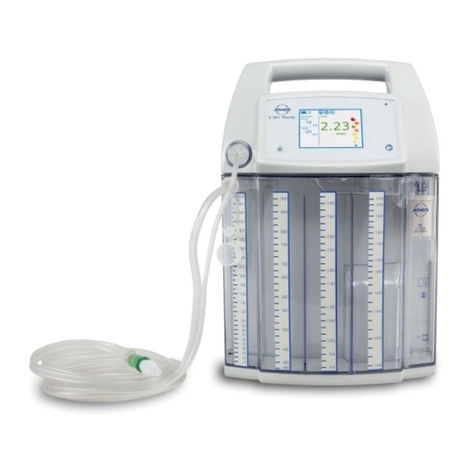
6
●Please observe the ambient conditions stated in the tech-
nical data (chapter 10.0).
●Always set up the device in such a way that the operating
elements are in clear view and within easy reach of the
operator. The device must be placed on a stable, level
surface.
●The ATMOS C 361 fully complies with the electromag-
netic immunity requirements of standard IEC 601-1-2 /
EN 60601-1-2 „Electromagnetic compatibility - Medical
ElectricalDevices“.
●The warranty period for this device is 2 years. This period
isunaectedbyanyrepairormaintenancecarriedout.
Please also pay attention to the enclosed General Terms
and Conditions.
●There are no warranty claims whatsoever on defects or
malfunctions which arise from the use of third-party ac-
cessories or consumables.
●ATMOS is not liable for personal injury and damage to
property if
• no original ATMOS parts are being used,
• the advice for use in these operating instructions is not
being observed,
• assembly, new settings, alterations, extensions and
repairs have been carried out by personnel not author
ised by ATMOS.
●This product is not re-sterilisable. Repeated reuse of
components which are marked with a 2is forbidden.
In case of repeated reuse these components lose their
function and there is a high infection risk.
●PleasedonotstoreDDSbacterialandviralltersunder
heavy objects since this may lead to deformation and
with it to loss of function.
There is a risk of contamination for the device.
●ATMOS recommends always having another suction
device ready to hand. That way you can perform
suctioning even in the event of device failure.
●The design of the ATMOS C 361fullstherequirementsof
IEC 601/ EN 60601. It is assigned to VDE protection class
II. It may only be connected to a properly installed earthed
power outlet.
●Before putting the device into operation, visually check the
unit, secretion canister, power cable, accessories, con-
nection cables and hoses for signs of damage. Damaged
cables and hoses must be replaced immediately. Check
function of the device prior to use.
●The ATMOS C 361 may only be used in supervised operation
byqualiedpersonnel(IEC601-1/EN60601-1).
●The device may be operated only in rooms used for medical
purposes. The ATMOS C 361 is not designed for use in
explosion-hazardous areas and in oxygen rich environ-
ments. Explosion-hazardous areas may be caused by the
use of ammable anaesthetics, skin cleansing products
and skin disinfectants.
●Do not allow any liquid to get into the device. If liquids have
penetrated the device, it may not be operated again until
it has been checked by the customer service centre.
●After transporting the device at temperatures below 0 °C,
keep it at room temperature for at least six hours before
initial start-up. If the device is not acclimatized it may not
be used as damage to the diaphragms of the pump could
occur.
●Dispose of wrappings accordingly.
●Before connecting the device , it must be checked whether
the required mains voltage and frequency on the device
matches the voltage and frequency ratings of the power
line.
●Only proper and undamaged plugs and extension cables
may be used.
●The suction hose must never come into direct contact
with the application site. A suction catheter, attachment or
a medical aspiration set must always be connected to the
hose.
● Whenusingdierentsecretioncanistersystemsthereis
a risk of contamination when operating the device without
an oversuction stop / hydrophobic DDS bacterial and viral
lter.
Do not use the device or rinsing canister without a DDS
bacterialandvirallter.
●There is a risk of an electric shock in the event of oversuc-
tion of the oversuction stop / hydrophobic DDS bacterial
andvirallter.
● Todisconnectthedevicefromthemainssupply,rstre-
move the plug from the wall outlet. Then disconnect the
power cable from the device. Never touch plug or cables
with wet hands.
2.0 For your safety