2
Table of contents
1 Introduction.......................................................................................................4
1.1 Notes on operating instructions ..............................................................................4
1.2 Explanation of pictures and symbols ......................................................................5
1.3 Intended use...............................................................................................................6
1.4 Function.......................................................................................................................8
1.5 Scope of delivery........................................................................................................8
1.6 Transport and storage...............................................................................................9
2 Notes for your safety......................................................................................10
2.1 General safety instructions.................................................................................... 10
2.2 Danger for users, patients, and third parties ...................................................... 10
2.3 Avoiding damage to the device ............................................................................. 11
3 Setting up and starting up .............................................................................12
3.1 Device overview....................................................................................................... 12
3.2 Preparing the device............................................................................................... 12
4 Operation.........................................................................................................13
4.1 Ambient conditions during operation .................................................................. 13
4.2 Switchingonthedevice(ATMOSS61CORIAN®integral)................................... 13
4.3 Switchingothedevice(ATMOSS61CORIAN®integral)................................... 13
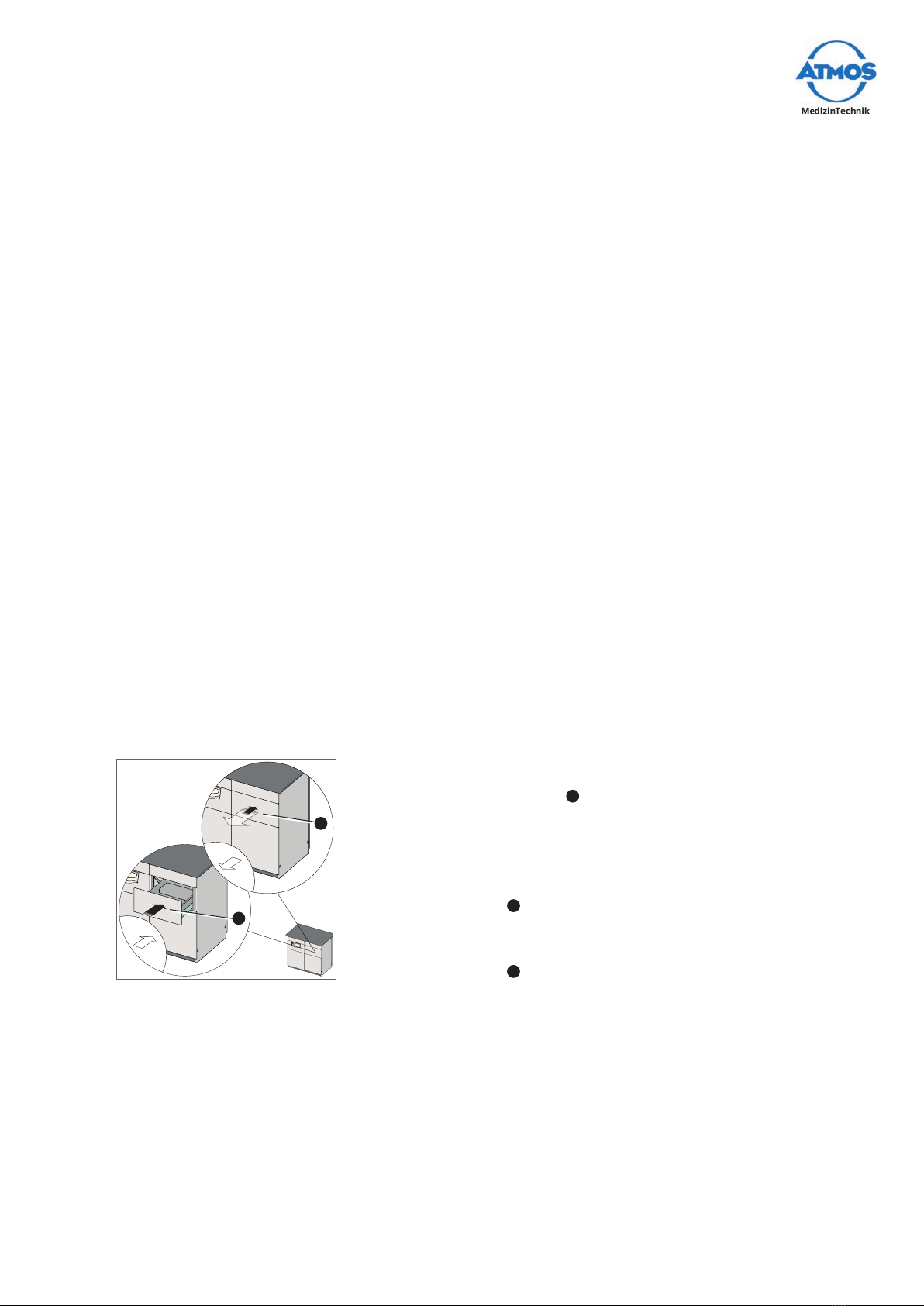
4.4 Using the drawers................................................................................................... 13
4.5 Usingthedrawerfordevices(optional) ............................................................... 14
4.6 Insertingandremovingtheinstrumentdisposaltray(optional) ...................... 14
4.7 Usingthewastedisposal(optional)...................................................................... 14
4.8 Usinglightsources(optional) ................................................................................ 15
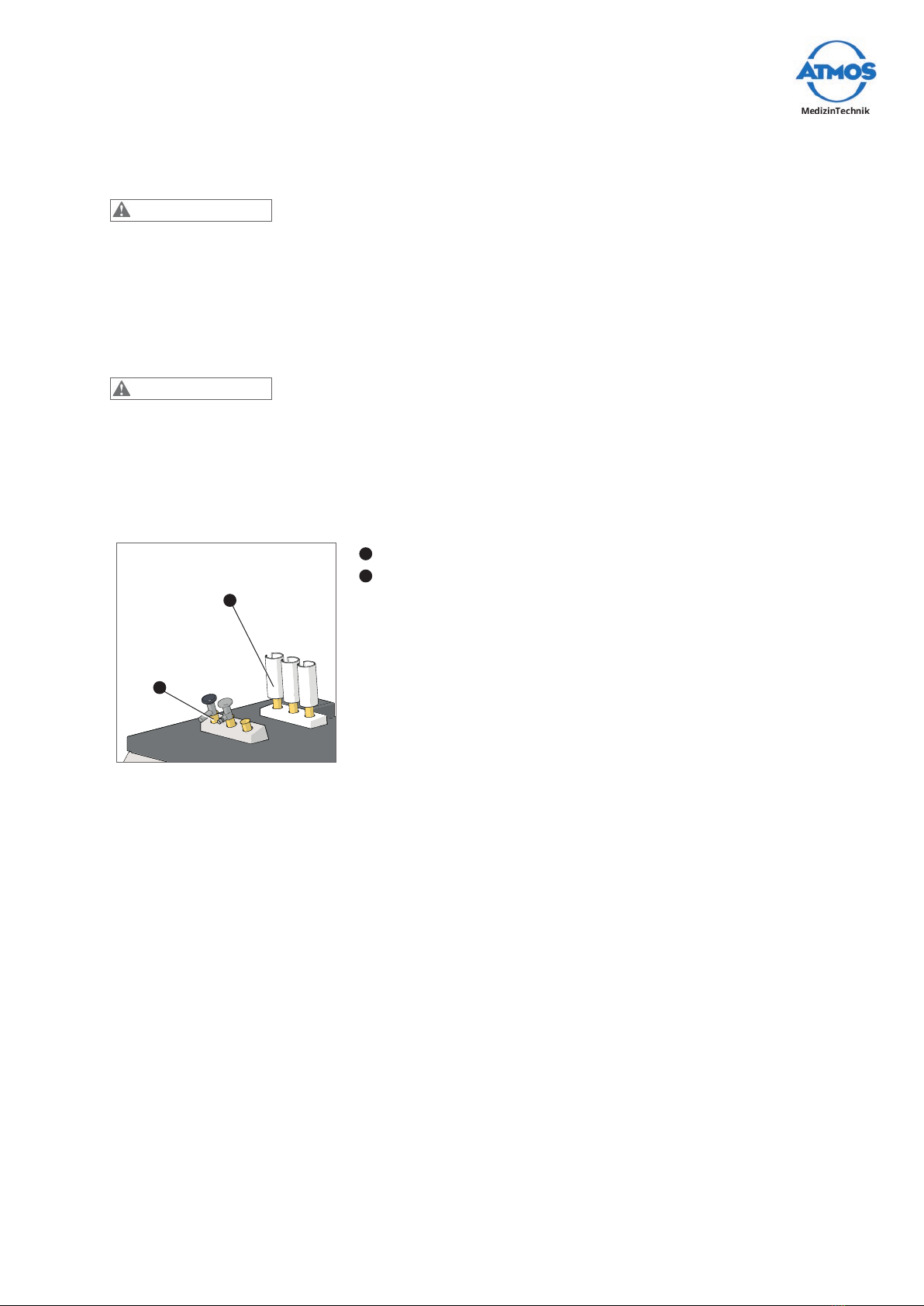
4.8.1 Connectingtheberopticlightcable ............................................................. 15
4.8.2 Removingtheberopticlightcable................................................................ 15
4.8.3 Changing the adapter........................................................................................ 15
4.8.4 Switching on the light source ........................................................................... 15
4.8.5 Adjusting brightness.......................................................................................... 15
4.8.6 Stroboscopycapabilityofthelightsource(optional).................................... 16
4.9 Storingendoscopes(optional)............................................................................... 17
5 Cleaning and disinfection ..............................................................................18
5.1 Reprocessing the product ...................................................................................... 18
5.1.1 Reprocessing the endoscope quivers ............................................................. 18
5.1.2 Reprocessing the instrument disposal trays .................................................. 18
5.1.3 Reprocessing the instrument trays ................................................................. 19
5.1.4 Reprocessingtheberopticlightcables ........................................................ 19
5.1.5 Reprocessing the surfaces................................................................................ 19
5.2 Recommended disinfectants................................................................................. 20
5.2.1 Surface disinfectants ......................................................................................... 20
5.2.2 Instrument disinfectants................................................................................... 21
6 Hygiene plan....................................................................................................23