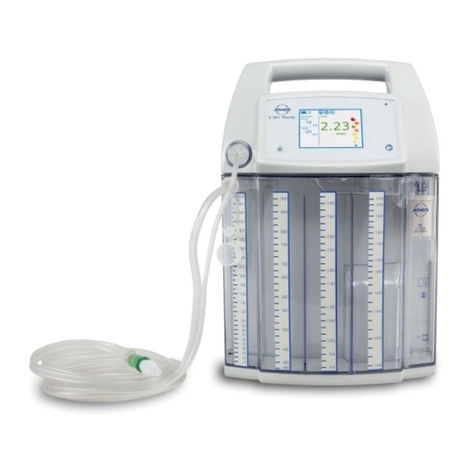
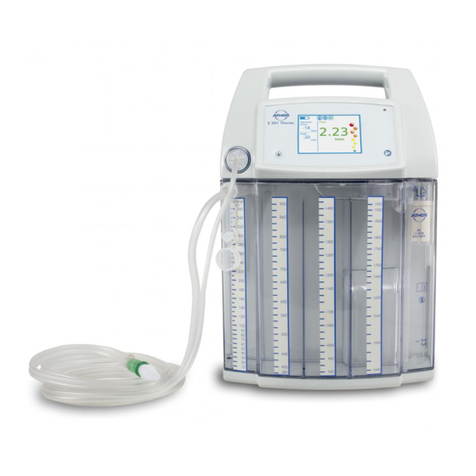
5
• The vacuum must be built up slowly in a controlled
manner for vacuum extraction.
• The user must continuously check the vacuum during
vacuum extraction.
• If the vacuum cannot be reduced despite correct
adjustment of the equipment (defective auxiliary air
valve), we recommend closing the suction hose between
pumpconnectionandbacteriallterinanair-tightmanner
(pinch) and then removing the suction hose from the
pump connection or cutting through the suction hose on
the pump connection with a scalpel. Then carefully let air
back into the suction hose (carefully release the pinched
suction hose) so that the vacuum is removed as slowly
as possible.
• The ATMOS®S 351 Natal version for vacuum extraction
ontrolley320.0070.0mustnotbeusedasuidsuction
unit for operations as the electronic secretion canister
overowmonitoronthistrolleydoesnotwork.
• The buffer canister for vacuum extraction must have a
volume of at least 1 litre.
• During vacuum extraction, the automatic mode can be
disabled by operating the foot controller.
• Prior to and during vacuum extraction, pay attention that
connection hoses are not kinked and that no blocked
lterisused.Priortoeachapplication,checkwhetherthe
lterisblocked.
• Vacuum extraction may not be possible at elevated
altitudes as it may not be possible to achieve the vacuum
required. It is at the discretion of the specialist whether
an operation using the suction unit can be carried out at
thenalvacuumobtained.
• Thecanisteroverowsafetyisdeactivatedduring
vacuum extraction.
• In case of a failure of the mains power supply or
accidental switching off of the equipment during
extraction, this must be aborted and production of the
vacuum has to be re-started after the equipment has
been successfully restarted. The best way to do this is by
pinching the suction hose to maintain the vacuum in the
cup, venting by pressing the END button and building the
vacuum again (pressing the extraction cup button) and
then applying the vacuum by releasing the pinched hose.
• Only approved extraction cups with CE marking in
accordance with RL 93/42 must be used.
• The system must not be ventilated suddenly with
simultaneous pulling on the extraction cup.
• Ifthenalvacuumsetisnotachieved,theATMOS®S
351Natalwillnotissueanaudiblesignal„nalvacuum
achieved“.
• The treating doctor is responsible for the proper surgical
procedure and technology! The adequacy and the kind of
application must be decided by a trained doctor according
to circumstances.
• The ATMOS®S 351 Natal may be used only by trained
staff under supervision (IEC 601-1 / EN 60601-1).
• The ATMOS®S 351 Natal meets the immunity to
interference requirements of IEC 601-1-2 / EN 60601-
1-2 „Electromagnetic Compatibility – Medical Electrical
Devices“.
• Always set up the unit in such a way that the operating
elements are in clear view and within easy reach of
the operator. Pay attention to maximum stability of the
installation surface.
• The ATMOS®S 351 Natal has been designed in
accordance with IEC 601/ EN 60601. The equipment
conforms to VDE Safety Class I. It must only be
connected to a properly installed earthed socket.
• Before connecting the device it needs to be checked
whether the requested mains voltage of the device
matches the mains voltage of the mains power supply.
• Use only correctly installed mains sockets and extension
cables.
• Check device, secretion canister, power cable,
accessories, connection cables and hoses for damage
before start-up. Defect cables and hoses must be
replaced immediately. Check functions of the device prior
to using it!
• Todisconnectthedevicefromthemainssupply,rst
remove the plug from the wall outlet. Disconnect the
connection line on the device afterwards only. Never
touch plug or line with wet hands.
• Following transportation at low temperatures the
appliance must be held for up to six hours at ambient
temperaturebeforerststart-up.Ifthedeviceisnot
acclimatized it may not be used as damages to the
electronic components could occur.
• When switching on the unit, a high vacuum value might
be present.
• This product is not re-sterilizable. Repeated reuse of
components which are marked with a 2is forbidden.
In case of repeated reuse these components lose their
function and there is a high infection risk.
2.0 For your safety