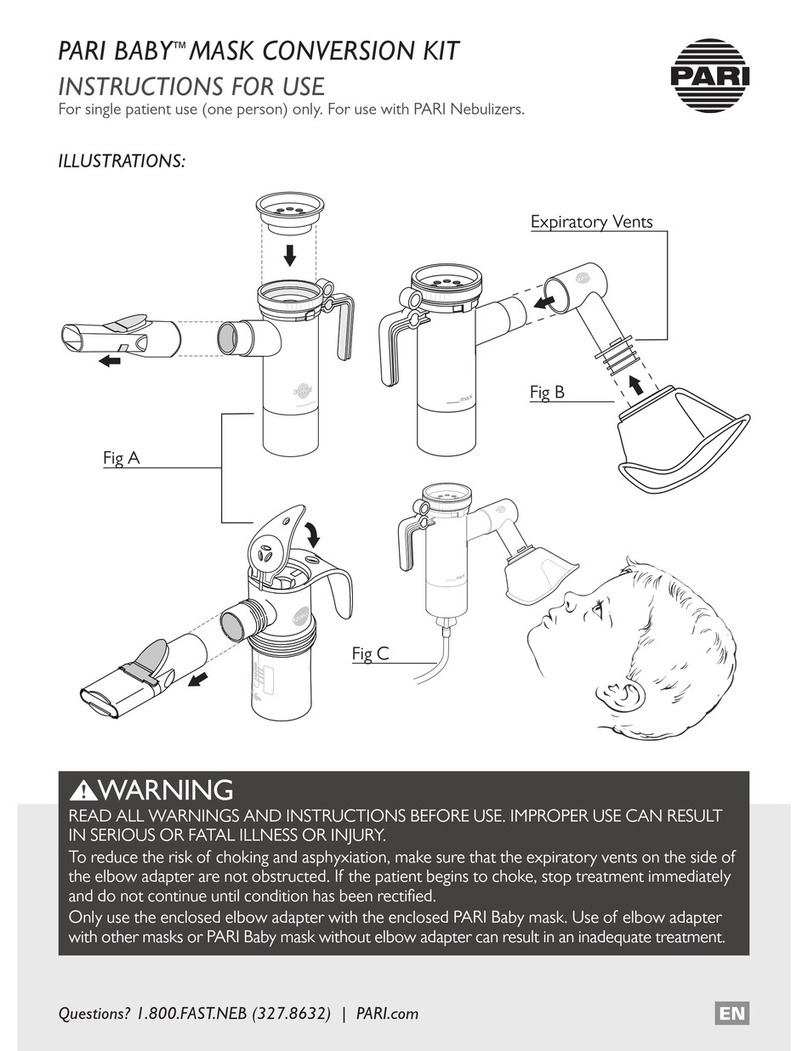
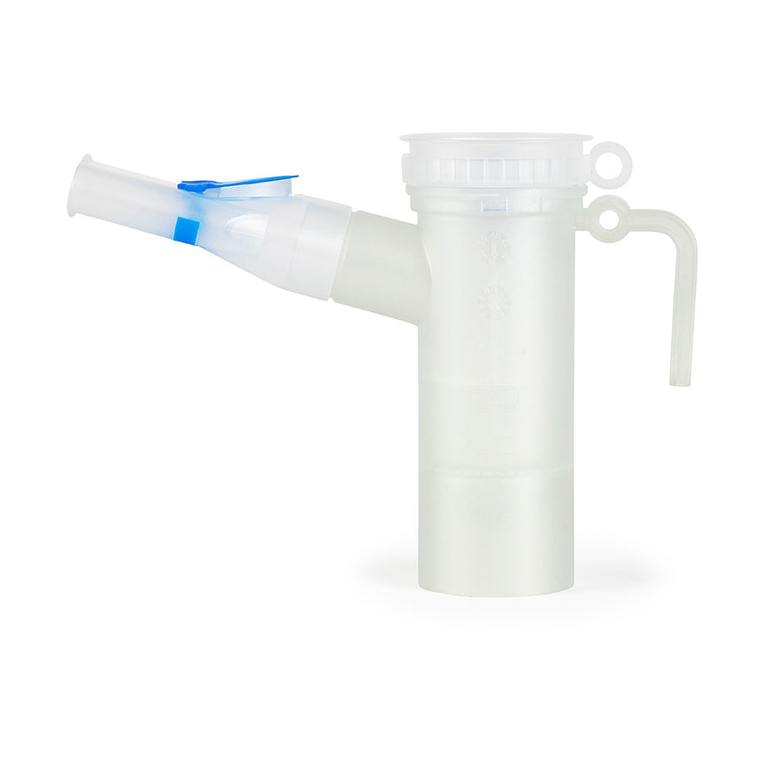
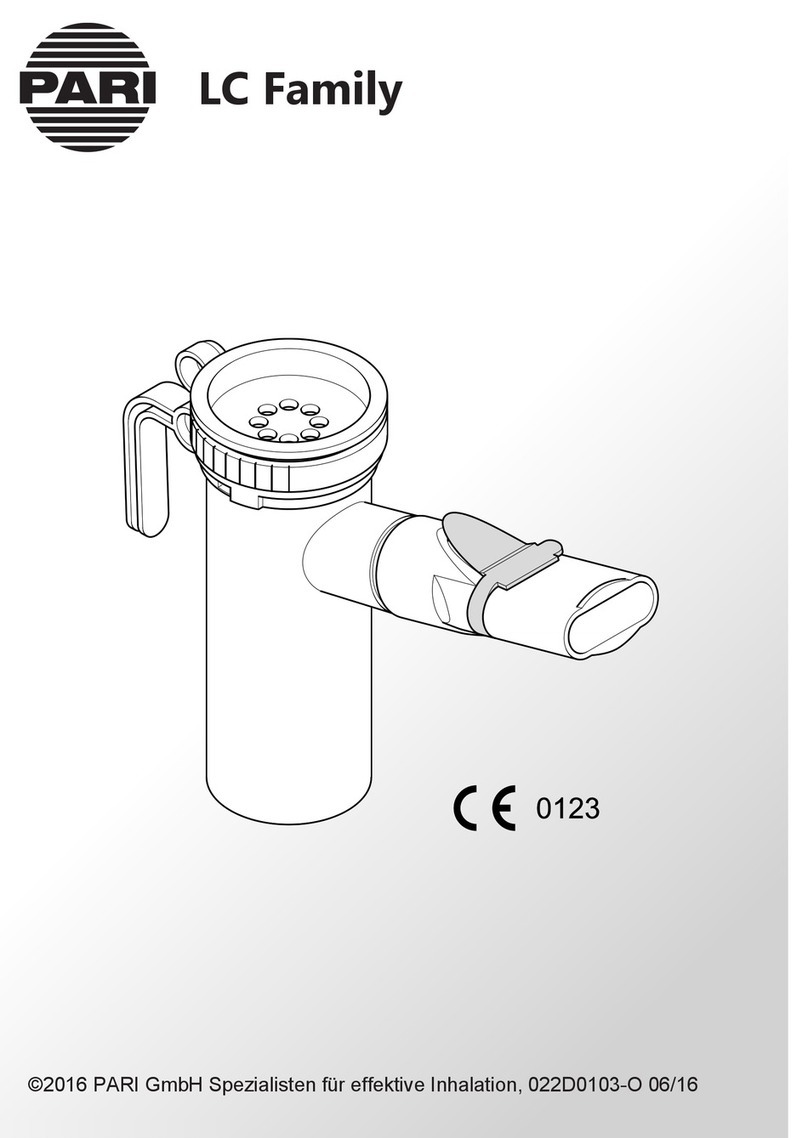
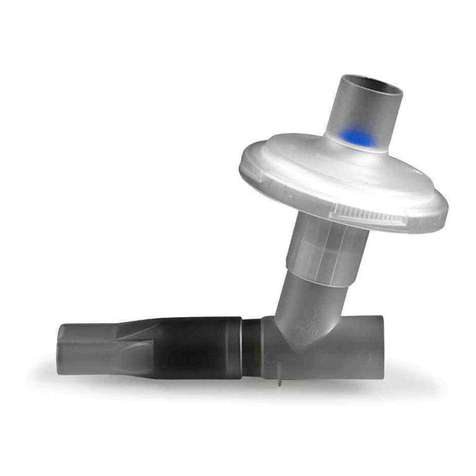
1 IMPORTANT INFORMATION
1.1 Intended purpose
The PARILCPLUS nebuliser generates inhalable aerosols1 for
treating the lower airways.
Together with a PARIcompressor or the PARICENTRAL and
with PARIaccessories, the nebuliser forms an inhalation sys-
tem.
The nebuliser is suitable for use in treating patients in all age
groups.
Only solutions and suspensions that are approved for use in
nebuliser treatment may be used.
The nebuliser must only be connected with a PARIcompressor
or with a central gas supply system. The PARICENTRAL is in-
tended for the connection with the central gas supply system.
This PARIproduct can be used in a home environment, as well
as in professional health institutions. When used in a home en-
vironment, this PARIproduct is intended for single-patient use
only (no patient change). In a professional environment, the
device can be used with different patients as long as the cor-
responding hygiene reprocessing measures are complied with.
This product must be used only by individuals who understand
the contents of the instructions for use and are able to use the
product safely. Individuals in the following groups must be su-
pervised by a person who is responsible for their safety:
– Babies, infants, and children
– Individuals with limited capabilities (e.g. physical, mental,
sensory)
If the patient is not able to use this product safely on their own,
then the treatment must be carried out by the responsible per-
son.
This PARIproduct is suitable only for patients who are able to
breathe by themselves and are conscious.
1) Aerosol: Small particles of solid, liquid, or mixed composition (fine “mist”)
suspended in gases or air.
– 5 –