9
Guidance and Manufacturer’s Declaration – Immunity
All Equipment and Systems
Table 6: Guidance and Manufacturer’s Declaration – Immunity
The BD EleVation™ Breast Biopsy System is intended for use in the electromagnetic environment specified below.
The user of the BD EleVation™ Breast Biopsy System should ensure that it is used in such an environment.
Immunity Test EN/IEC 60601 Test Level Compliance
Level
Electromagnetic Environment –
Guidance
ESD EN/
IEC 61000-4-2
+/-2kV, +/-4kV, +/-8kV
Contact discharge
+/- 2kV, +/-4kV, +/-8kV,
+/-15kV
Air discharge
Pass
Floors should be wood, concrete or
ceramic tile. If floors are synthetic, the r/h
should be at least 30%.
EFT EN/
IEC 61000-4-4
+/-2 kV, 100 kHz PRF
+/-1 kV, 100 kHz PRF Pass
Mains power quality should be that
of a typical commercial or hospital
environment.
Surge EN/
IED 61000-4-5 +/-0.5 kV, +/-1 kV Pass
Mains power quality should be that
of a typical commercial or hospital
environment.
Voltage Dips/
Dropout
EN/IEC 61000-
4-11
100% dip for 0.5 Cycle at
0°, 45°, 90°, 135°, 180°,
225°, 270° and 315°
100% dip , 1 cycle
30% dip, 25/30 cycles
100% Dip for 250/300
cycles
Pass
Pass
Pass
Pass
Mains power quality should be that
of a typical commercial or hospital
environment. If the user of the BD
EleVation™ Breast Biopsy System requires
continued operation during power mains
interruptions, it is recommended that the
BD EleVation™ Breast Biopsy System be
powered from an uninterruptible power
supply or battery.
Power Frequency
50/60Hz
Magnetic Field
EN/IEC 61000-4-8
30A/m Pass
Power frequency magnetic fields should
be that of a typical commercial or
hospital environment.
Guidance and Manufacturer’s Declaration – Immunity
Equipment and Systems which are NOT Life-supporting
Table 7: Guidance and Manufacturer’s Declaration – Immunity
The BD EleVation™ Breast Biopsy System is intended for use in the electromagnetic environment specified below.
The user of the BD EleVation™ Breast Biopsy System should ensure that it is used in such an environment.
Immunity Test EN/IEC 60601 Test Level Compliance
Level
Electromagnetic Environment
Guidance
Conducted RF EN/
IEC 61000-4-6
Radiated RF EN/
IEC 61000-4-3
3 Vrms 150 kHz to
80 MHz
3 V/m 80 MHz to 2.7GHz
Pass
Pass
Portable and mobile communications
equipment should be separated from the
BD EleVation™ Breast Biopsy System by
no less than the distances calculated/
listed below:
D=(3.5/V1)(√P)
D=(3.5/E1)(√P) 80 to 800 MHz.
D=(7/E1)(√P) 800 MHz to 2.7GHz
Where P is the max power in watts and D
is the recommended separation distance
in meters.
Field strengths from fixed transmitters,
as determined by an electromagnetic
site survey, should be less than the
compliance levels (V1 and E1).
Interference may occur in the vicinity of
equipment containing a transmitter.
Guidance and Manufacturer’s Declaration – Emissions
Equipment and Systems which are NOT Life-supporting
Table 8: Recommended Separations Distances for the BD EleVation™ Breast Biopsy System
The BD EleVation™ Breast Biopsy System is intended for use in the electromagnetic environment in which
radiated disturbances are controlled. The customer or user of the BD EleVation™ Breast Biopsy System can help
prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF
Communications Equipment and the BD EleVation™ Breast Biopsy System as recommended below, according to
the maximum output power of the communications equipment.
Max Output Power
(Watts)
Separation (m)
150kHz to 80MHz
D=(3.5/V1)(√P)
Separation (m)
80MHz to 800MHz
D=(3.5/E1)(√P)
Separation (m)
800MHz to 2.7GHz
D=(7/E1)(√P)
0.01 0.1166 0.1166 0.2333
0.1 0.3689 0.3689 0.7378
1 1.1666 1.1666 2.3333
10 3.6893 3.6893 7.3786
100 11.6666 11.6666 23.3333
Recommended Separation Distances between portable and mobile RF Communications
equipment and the BD EleVation™ Breast Biopsy System Equipment and Systems which are
NOT Life-supporting
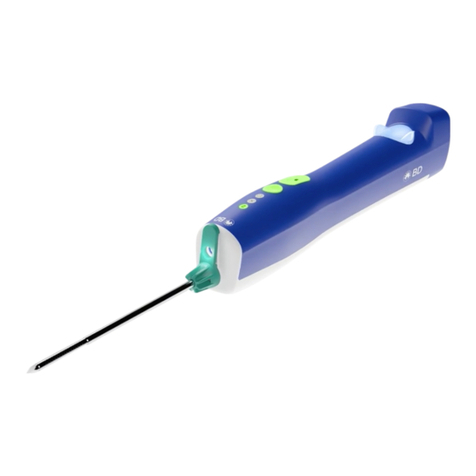
How Supplied
• The BD EleVation™ Driver is supplied with a wireless charging stand, AC cord, and AC power
adapter plugs. During unpacking, check for missing or damaged components.
• The BD EleVation™ Driver is supplied non-sterile and should be cleaned prior to each use.
• The BD EleVation™ Probes are sold separately. The BD EleVation™ Probes are supplied sterile
for single use only.
• The BD EleVation™ Accessories are sold separately. The BD EleVation™ Accessories are
supplied sterile for single use only.
Warranty
Bard Peripheral Vascular, Inc. warrants to the first purchaser of this product that this product will
be free from defects in materials and workmanship for a period of one year from the date of first
purchase and liability under this limited product warranty will be limited to repair or replacement
of the defective product, in Bard Peripheral Vascular, Inc.’s sole discretion or refunding your net
price paid. Wear and tear from normal use or defects resulting from misuse of this product are not
covered by this limited warranty.