BIOMET EBI Bone Healing System User manual

The Biomet®EBI Bone Healing System
Physician Manual and Package Insert
English Language
399JeffersonRoad,Parsippany,NJ07054
800.526.2579•www.biomet.com•1068220-00Rev.A•BNS231188L
©2013EBI,LLC.Allrightsreserved.AlltrademarksarethepropertyofBiomet,Inc.
oroneofitssubsidiariesunlessotherwiseindicated.†denotes a third party trademark. Velcro®is a registered
trademarkofVelcroIndustriesandVelcroUSAInc.RxOnly.PrescriptionOnly-SingleUseOnly-
NotforRe-saleorRe-Distribution-DoNotReuse

Contents
ImportantSafeguardsandContents ................................. Page 1
Biomet®EBI Bone Healing System...................................Page2
Description....................................................................Page2
Electrical Requirements ................................................Page2
SystemComponents......................................................... Page 3
FullPrescribingInformation..............................................Page4
IndicationsforUse........................................................Page4
Contraindications ..........................................................Page4
Warnings,Precautions,AdverseEffects.......................Page5
General Treatment Instructions.........................................Page6
OperatingInstructions....................................................... Page 8
Step1:BatteryCharging............................................... Page 8
Step2:PreparingtheSystemtoBeginTreatment....... Page 9
Step3:TreatingandCharging...................................... Page 9
Step4:RechargingtheBatteries ..................................Page10
ControllerHolder........................................................... Page 11
KeypadFunctions..........................................................Page12
Troubleshooting System Messages.............................. Page 13
ComplianceDataSoftware............................................Page14
SFLXFlexibleTreatmentCoils...........................................Page17
SFLX-1,SFLX-2,SFLX-3,SFLX-4
andSFLX-5CoilApplication ............................................. Page 18
ConformingtheSFLXTreatmentCoil........................... Page 19
CastedandNoncastedApplications .............................Page20
CleaningInstructions ....................................................Page21
TreatmentCompletion.......................................................Page21
ReturningDefectiveProduct .............................................Page22
EquipmentClassification...............................................Page22
Disposal/Recycling .................................................... Page22
SymbolDescription.......................................................Page22
OrderingInformation.........................................................Page23
References.........................................................................Page23
Biomet®EBIBoneHealingSystemComponents..............Page24
ElectromagneticCompatibility...........................................Page25
FurtherInformation ...........................................................Page29
ReimbursementForm .......................................................Page30
1
Important Safeguards and Contents
Read All Instructions Before Using
Whenusingelectricalproducts,basicsafety
precautions should always be observed:
! WARNING:Toreducetheriskofelectricshock,
fireorpotentialinjuriespleaseadheretothe
following:
1. Donotuseyourbonehealingsystemwhile
bathing.
2. Donotplaceorstoreyourbonehealing
systemwhereitcanfallorbepulledintoa
tub,sinkoranypoolofliquid.
3. Donotimmerseordropyourbonehealing
system’scontrolunit,treatmentcoil,AC
WallAdapter,inanyliquid.
4. Donotreachforyourbonehealingsystem
thathasfallenintoaliquid.Unplugfrom
the wall outlet immediately.
5. DonotpermittheACWallAdaptertobe
connected when wet.
6. NevertouchtheACWallAdaptercontacts
whentheACWallAdapterispluggedinto
anACWalloutlet.
7. DonotplacetheACWallAdapterinthe
bedwithyouifyouaretreatingwhileyou
are sleeping.
8. Neveroperateyourbonehealingsystemif
it has a damaged link cable, cord or plug,
ifitisnotworkingproperly,ifithasbeen
dropped and damaged, or immersed into
anyliquid.ContactBiometimmediatelyfor
a replacement part(s).
9. Keep all electrical cords and cables away
fromheatedsurfaces.
10. Keepallelectricalcordsandcablesaway
fromchildren.
11. Neverinsertanyobjectintoanyopeningof
your bone healing system.
12. Donotplaceyourbonehealingsystem’s
control unit in prolonged heat or direct
sunlight.(Normaloperatingtemperature
rangeis5°Cto38°C,[41°Fto100°F],
20-80%RHnon-condensing,normal
storage/transporttemperatureis-15°Cto
60°C[5°Fto140°F].)
13. Useyourbonehealingsystemonlyforits
intended use as prescribed by your
physician and described in this manual.
14. Nomodificationtothisdeviceisallowedfor
any reason whatsoever.
15. Routineuseofbonehealingsystemsfor
over30yearshasdemonstratedthatany
known hazard associated with their use
doesnotpresentanunreasonableriskof
illnessorinjurywhencomparedtothe
benefitoftheiruse.
16. Useofyourbonehealingsystemforthe
spine and skull has not been evaluated or
approved.
*NOTE: Please contact the Biomet Patient
SupportDepartmentinParsippany,NewJersey
betweenthehoursof8:30a.m.and5:30p.m.
EasternTimeat1.973.299.9300withany
questions or problems.
SAVE THESE INSTRUCTIONS
Contents – Biomet®EBI Bone Healing System
Controlunit/Controller
Connectorcables(0"and28")
Belt
ClipHolder
ACWallAdapter
UserManualandPackageInsert
Carryingcase
CAUTION:RxOnlyFederalLaw(U.S.A.)restricts
thisdevicetosalebyorontheorderofa
physician.ForPrescriptionUseOnly.Single
prescription.Singlepatientuse.Notforre-sale.
Please note: This system does not contain a
treatmentcoil.PleasecontactyourBiomet/
EBIrepresentativeforthespecificanatomically
correct treatment coil.
*NOTE:TheControlUnitmustnotbewornon
the coil.
2
Biomet®EBI Bone Healing System
SFLX Treatment Coil
Description
The Biomet®EBI Bone Healing System
promotes healing by inducing weak pulsing
electricalcurrentsatthefracturenonunion
site. These signals are generated by a low
energyelectromagneticfieldcreatedbypassing
specificcurrentpulsesthroughananatomically
configuredtreatmentcoil.
Electrical Requirements of AC Wall Adapter
Unit – USA/Americas
Input:100–240V~50–60Hz0.3-0.8A
Output:12V @2.0AMAX
DonotuseanyotherACwalladapterwiththe
Biomet®EBI Bone Healing System.
Electrical Requirements
The Biomet®EBIBoneHealingSystemControl
unitisavailableinthefollowingconfiguration.
Americas
• IncludesanACAdapterchargerwitha
standard two prong plug
*NOTE: AnInternationalWallPlugBlade
AdapterKitisavailableforpatientswhowillbe
travelingoutsidetheUSandwishtocontinue
their treatment while abroad.

3
System Components
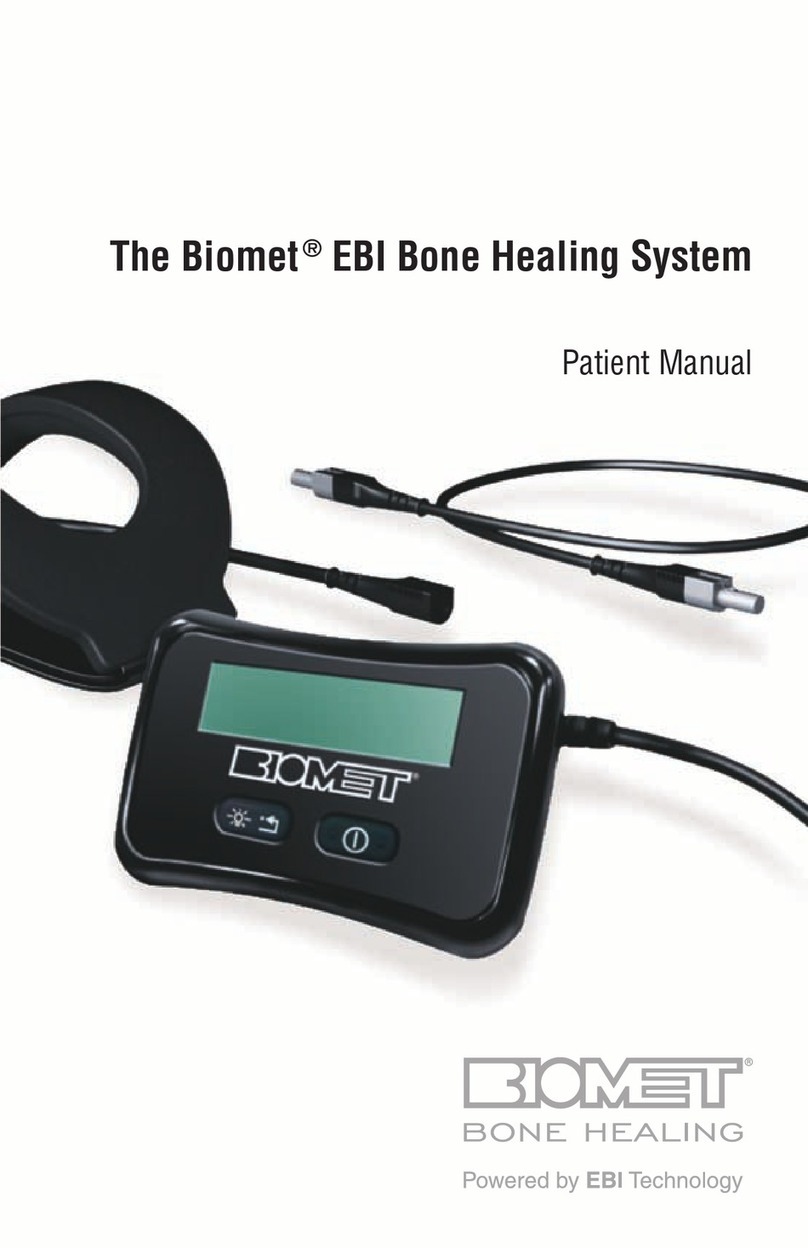
Control Unit – Controller
The Biomet®EBI Bone Healing System control
unit operates on a permanently installed lithium
ion,rechargeablebatterywhichfacilitates
ambulatory use. The control unit contains solid
state electronics programmed to operate the
SFLXfamilyofTreatmentCoils.Itincludes
anaudibleandvisibleselfcheckingalarm
mechanismtoalertthepatientiftheunitisnot
functioningproperly.
*NOTE: The control unit can not be used with
theFLX®familyofTreatmentCoilsusedwith
the EBI Bone Healing System®-Model2001.
The control unit is designed to store the
patient’sdailytreatmentinformation.Patients
are recommended to bring the control unit and
treatmentcoiltoeachfollow-upvisittoprovide
the opportunity to review their overall treatment
complinace in how they are using their Biomet®
EBI Bone Healing System.
*NOTE:Whilewalking,thepatientmaywearthe
controlunitcomfortablyonabeltorthewaist
usingtheClipHolder.
Battery Charger/AC Wall Adapter
1. TheACWallAdapterisdesignedto
recharge the permanently installed battery
inside the control unit. (See step 3: Treating
andCharging–page9)
2. Thecontrolunitallowsfortreatingwhile
charging.
DonotuseanyotherACWallAdapterwiththe
Biomet®EBI Bone Healing System.
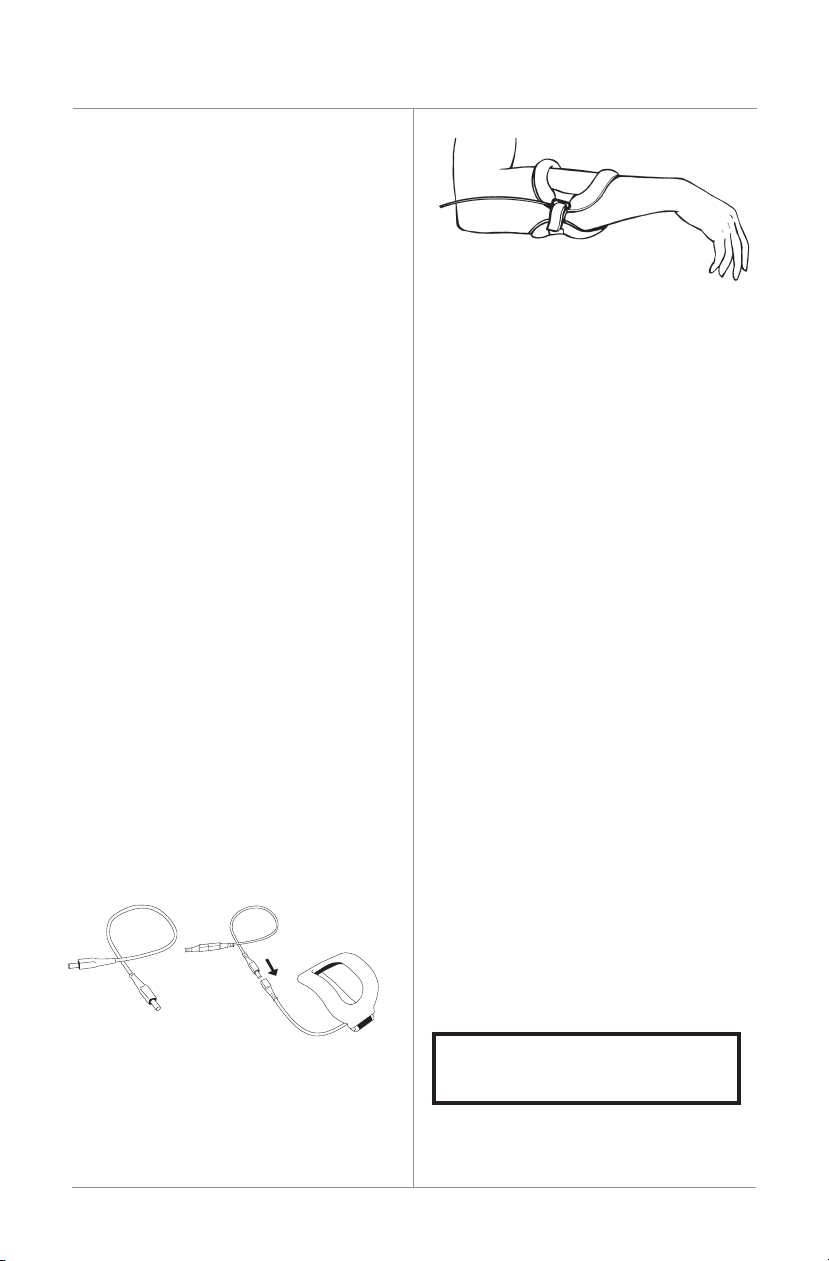
Link Cable
The link cable connects the control unit to its
SFLXFlexibleTreatmentCoil.Thelinkcables
suppliedare“zero”and28"cables.Anoptional
linkcableisalsoavailableina48"length.For
a48"cable, call the Biomet Patient Support
Departmentat1.973.299.9300.Outsidethe
UnitedStatescontactyourlocalEBI/Biomet
Distributor.
SFLX Flexible Treatment Coil
TheSFLXFlexibleTreatmentCoilisanencased
wire coil that may be incorporated into a cast,
over a cast or brace, or when a cast is not
utilized, may be applied directly onto the skin.
Aspecificelectricalcurrentisdeliveredtothe
coil by the control unit. The coil then delivers
the therapeutic electromagnetic signal to the
fracturenonunionsite.
*NOTE: TheSFLXCoilisnotincludedwith
the system assembly and will be provided
separately by your Biomet sales representative.
*NOTE:TheControlUnitmustnotbeworn
on the coil.
Controller
Coil
Cable
4
Full Prescribing Information
ThemechanismofactionbehindthePEMF
technologyinvolvestheupregulationoffactors
thatmodulatenormalbonehealing.PEMF
increasesanumberoffactorssuchasTGF-
β1,BMP-2andBMP-4,whicharenormal
physiologicalregulatorsofthevariousstages
ofbonehealing,includingangiogenesis,
chondrogenesis and osteogenesis.
Intended User Profile
The EBI Bone Healing System®is intended to
treat adults who have been medically diagnosed
withafracturenonunionorfailedfusioninthe
appendicular system. It is also intended to treat
congenital pseudarthrosis in the appendicular
sytem in pediatric patients not skeletally
mature.
Indications for Use
The Biomet®EBI Bone Healing System
isindicatedforthetreatmentoffracture
nonunions,failedfusions,andcongenital
pseudarthrosisintheappendicularsystem.A
nonunion is considered to be established when
there are no visibly progressive signs
ofhealing.
Theoriginal1979PMAstudyincluded146
patientswithnonunionfractures.Thesedifficult
fractureswerecharacterizedasfollows:2.3
averagenumberofpriorsurgeriesandan
averageofthirty-sevenmonths(median
twentymonths)sinceoriginalinjury.These
patientswerefollowedforaminimumoffour
years(averagesevenyears)fromthedateof
treatment termination, with a success rate
of63.5%.Eventhoughlongtermfollow-up
requirements were not included in the original
studydesigns,afollow-uprateof82%was
achieved.Forty-three(43)oftheoriginal48
patients in the congenital pseudarthrosis study
wereclassifiedbyBassett1whodefinedthe
tibiallesionsasTypeI(n=6),TypeII(n=19)
and Type III (n=18), with Type III being the
most severe and recalcitrant to treatment. The
successrateforBassettTypeIlesionswas
66.7%,BassettTypeIIlesions57.9%and
BassettTypeIIIlesions22.2%.Thelongterm
posttreatmentfollow-upforthecongenital
pseudarthrosisstudypatientpopulation(n=48)
wastoskeletalmaturityortheageof18.The
studyhadan87.5%follow-uprate.
1. BassettCAL,NCauloandJKort,“Congenital
pseudarthrosisofthetibia:Treatmentwith
pulsingelectromagneticfields”.
ClinicalOrthop,154:136-149,1981.
Contraindications
A. Nonunionfracturesinwhichasynovial
pseudarthrosis(fluidfilledgap)exists.
B. Undercertainconditions,electromagnetic
stimulation could inhibit or impair the
functioningofcertainexternal,non-invasive
and/orimplanted,invasiveactivemedical
devicesinclusiveof“allactiveelectricaland
non-activeconductive/metallicimplants”
as well as “worn medical devices” due to
adverse events that may occur with other
activeelectricalimplants(e.g.,SpinalCord
stimulators,ImplantableCardioverter-
defibrillators,etc.)Theimpactoreffectof
pulsedelectromagneticfieldsgenerated
by a non invasive bone growth stimulator
onthefunctionofotheranatomical
stimulators, pain pumps, insulin pumps,
implanted spinal nerve stimulators and
similar active devices has not been
evaluated.
5
C. UseoftheBiomet®EBI Bone Healing
System on pregnant patients has not been
evaluated;therefore,itisnotrecommended
in these cases.
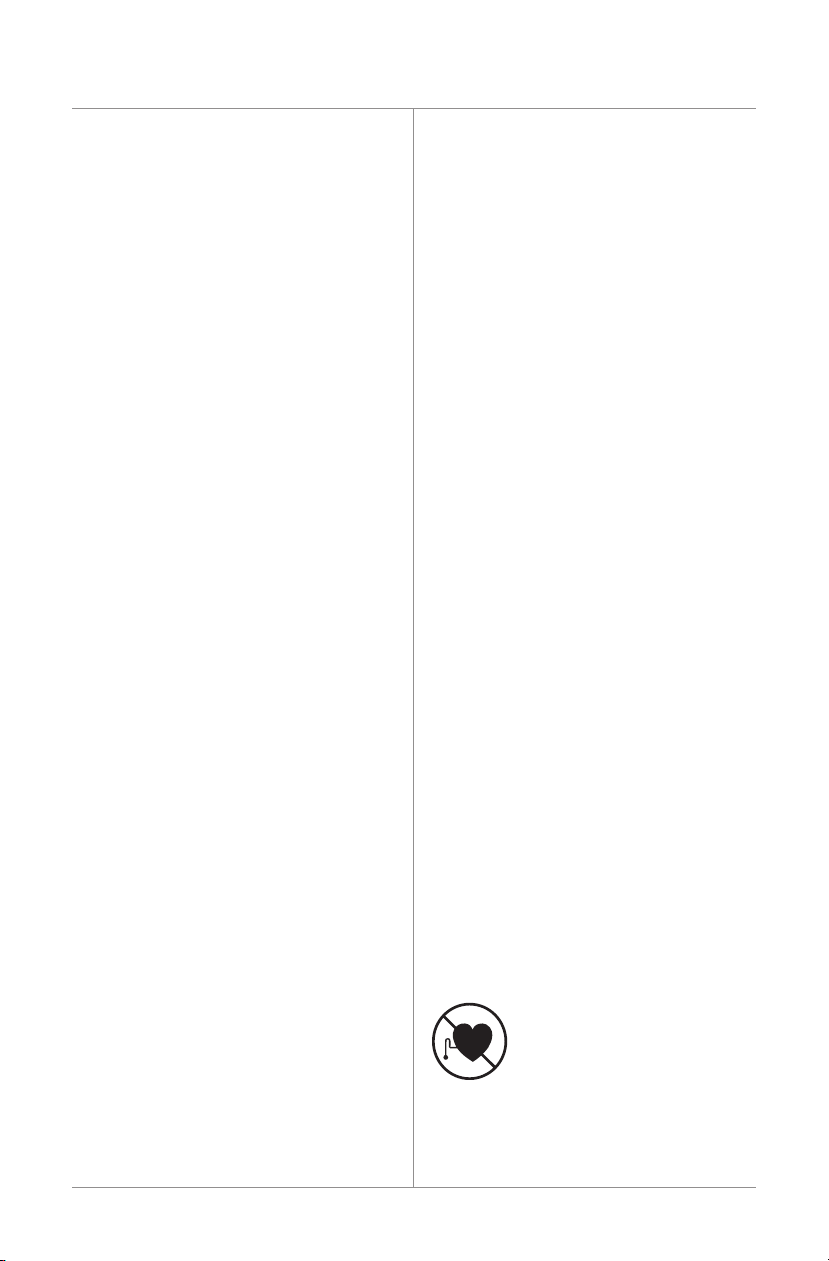
D. TheBiomet®EBI Bone Healing System has
notbeentestedforsafetyorbeenevaluated
forheatingintheMRenvironment.The
effectsofMRIproceduresandscansusing
MR systems has not been determined
orestablished:therefore,MRIscansand
proceduresshouldnotbeperformedon
patients until the device system has been
completely removed.
MRUnsafe-NotforMRIUse
Warnings
A. Thelongtermeffectsofexposureto
lowlevelmagneticfieldsarenotknown.
Routineuseofthebonehealingsystem
devicesforover30yearshasindicatedno
known risks.
B. Duringthetreatmentofpatientswithopen
epiphyses, when the epiphysis is in the
pulsingfield,physiciansareadvisedthat
the epiphyseal growth plates should
bemonitoredforpossibleeffects.
C. UseoftheBiomet®EBI Bone Healing
Systemforthespineandskullhavenot
been evaluated.
D. Toreducetheriskofpotentialinjury:
1. AVOIDtouchingtheACWallAdapter
contactswhentheACWallAdapteris
pluggedintoanACoutlet.
2. DONOTchargethebatteryinbedif
treating while sleeping.
E. The control unit is electrically live when
connectedwiththeACWallAdapterand
pluggedintoanoutlet.Toreducetheriskof
seriousinjurybyelectricshockpatientsare
advised:
1. DONOTpermittheACAdaptertobe
connected when wet.
2. DONOTimmersethecontrolunit,
treatmentcoil,ortheACWallAdapter
in any liquid.
Precautions
Thefollowingconditionsmaycompromisea
successfultreatmentoutcome.
A. Nonunionfractureswithgapsinexcess
of1.0cm.
B. Presenceoffixationdevicesor
instrumentationmadefrommagnetic
materials.
Please note: Most presently used internal
orexternalfixationdevicesareconstructed
of316LS.S.,titaniumalloys,andcobalt-
chromiumalloyswhicharenon-magneticand,
therefore,compatiblewiththeBiomet®EBI
Bone Healing System.
Adverse Effects
Based upon an historical adverse event report re-
viewcomposedofMedicalDeviceReports–MDRs
identifiedwithintheFDAManufacturerandUser
FacilityDatabaseforsimilar,relevantdevices,the
probabilityofexperiencinganadverseeventwhen
usingaBoneHealingSystemisextremelyunlikely,
orlessthan1%(.0133%).
The database, although imprecise is intended to
provideandpresentageneralsummaryofproduct-
specificeventdatathatmanufacturers,userfacilities
anddistributorsprovidetotheFDAbasedupon
relevantproductcodesforsimilardevices.
The original EBI Bone Healing System was approved
andintroducedin1979.Sincethen,over600,000
systemshavebeenmarketed.Thesafeandeffec-
tiveuseofnon-invasivebonegrowthstimulation
devices over this time has clearly established their
therapeuticbenefitofuse.Inaddition,allknown
hazardsassociatedwiththeuseofBoneHealing
Systemsdonotpresentanunreasonableriskof
illnessorinjurywhencomparedtotheirtherapeutic
benefitandcanbetypicallyaddressedbyeither
modifyingorterminatingtreatment.
6
General Treatment Instructions
Recommended Usage
The recommended daily treatment dosage is
normally10hoursperday.
Althoughthedevicewasshowntobeeffectiveat
treatmenttimesbetween3to10hoursperday,a
reviewoftheclinicaldatahasshownthathealing
may occur earlier when treatment is achieved at
therecommended10hoursperday.
Compliancewiththerecommendedten (10)
hoursperdaytreatmentisveryimportant.A
reviewoftheclinicaldatademonstratesthat
lessthantherecommendeduseofthisdevice
possibly results in an increase in the time to
healyourfracture.Ifyouareunabletotreatfor
ten continuous hours, it is recommended that
you break up the total treatment time into more
thanonesession.Pleaserefertothefollowing
General Treatment Instructions.
General Treatment Instructions
Treatment should not be suspended until
healing occurs or until such time as the
prescribing physician recommends
discontinuationofthedevice.
The Biomet®EBI Bone Healing System is
programmedtodeliveramaximumof270
therapeutic treatment periods. Biomet
recommends a therapeutic treatment period
of10hoursperday.Atherapeutictreatment
periodisdefinedasa10hourtreatment
session accomplished either continuously or
discontinuouslyinsegmentstotaling10hours.
• Unsegmented Continuous Treatment Period:
Patient wears the device with coil in place for
10 consecutive hours without interruption
•Segmented Discontinuous Treatment Period
(Example):Patient treats with coil in place for
four hours, turns the device off and does not
push the RESET button. Patient then turns the
device on within the next 12 hours and only
completes the remaining six hours of treatment
for that therapeutic treatment period.
•Shortened Segmented:
A treatment period of less than 10 hours may
be achieved by pushing the reset button prior
to the completion of the 10-hour period.
Once the device has been reset, the system
software will reset the controller to start a
new treatment period.
•Shortened Segment Treatment Period
(Example): Patient completes eight hours of
treatment with coil in place and then turns
device off. Less than 12 hours later, the patient
turns device on and pushes RESET button.
Patient received eight hours of treatment and
controller is set to treat for a full 10 hours.
Patient compliance usage log is credited with
eight hours.
Allhourstreatedwillbeelectronicallyrecorded
and stored within the controller. Patients unable
to treat on consecutive days will be able to treat
non-consecutivelywithin400daysafterthe
firstone-hourtreatmentperiod.Ifthedevice
isnotusedfora12hourperiod,thetreatment
clock will automatically reset and be ready to
deliverthenext10hourtherapeutictreatment
periodwhennextturnedon.
•Non-consecutive treatment period
(Example): On Monday, the patient treats for
seven hours and turns the device off. Patient
does not treat on Tuesday. Wednesday, patient
turns controller on. System will be ready to
treat for a full 10 hours, not the remaining three
from Monday’s period. Device usage log will
record a seven hour treatment and a day of no
treatment.
Themaximumrecommendedtherapeutic
treatmentperiodisninemonths(approximately
270days).
7
*Note:
•Thecontrolunitshouldbeturnedoffwhen
finishedwiththetreatmentsession
•Whenreadytoresumetreating,turnthe
control unit on. The display will indicate the
treatment time that has been completed and
keepstrackofthecumulativetreatmenttimefor
the day
•Ifthepatientdoesnotfinishtenhoursinthat
day, then use the RESET button to return the
time to zero
Forconvenience,thedailytreatmenttimeis
displayed continuously in hours and minutes.
Aftertenhoursoftreatment,thedisplaywill
read“10:00”,andbeepthree(3)times,and
thenshutoffautomatically.
ATTENTION: This is a single patient use device,
donotreuse.ForPrescriptionUseOnly.The
Biomet®EBI Bone Healing System is a durable
therapeuticelectricaldeviceintendedforsingle
patient use only under a prescription. Treatment
at home or in another appropriate or similar
setting is acceptable. The device cannot be
reprocessed,refurbished,disinfected,resold,
sterilized, etc. with the intent to be used by
anotherpatientorfortreatmentotherthan
initially prescribed.
This device has been designed to reduce the
riskofpotentialreusebyautomaticallyceasing
tofunctioninaccordancewiththeGeneral
TreatmentInstructionsspecifiedanddefined
within.
Recommended Concurrent Fracture
Treatment
The Biomet®EBI Bone Healing System works
bestwhenmotionofthefracturesiteis
minimizedornonexistent.Thisimmobilization
is achieved by applying a well molded plaster or
syntheticcastatthebeginningoftreatment
(togetherwithinitialnon-weightbearingifitis
treatingthelowerextremity.)
• Ankle/Tarsals/Metatarsals:shortlegcast
or rigid internalfixation
• Tibia:stableinternalorexternalfixation,
or long leg cast (short leg cast with rigid
fixation)
• Shoulder/Clavicle:braceorabductionsplint,
internalfixation,orfigureeightimmobilization
• Humerus:stableinternalfixationand/or
adequate immobilization with controlled
rotation
• Scaphoid/Wrist:stableinternalfixationor
long arm cast with thumb spira (short arm
castwithrigidfixation)
• Carpals/Metacarpals/Phalanges:cast,
internal,orexternalfixation
Operating Instructions
BeforeusingtheBiomet®EBI Bone Healing
Systemforthefirsttime,thepermanently
installed battery housed within the control unit
mustbefullycharged.
Step 1: Battery Charging
The Biomet®EBI Bone Healing System
operatesonalithiumionbattery.Before
treating with the system,thepatientmustfully
chargethebattery.Atroomtemperatures,
(24°C[75°F])chargingmaytakeuptothree
andone-halfhours.
Controller
A. PlugtheACAdapterintoanACwalloutlet.
B. ConnecttheACWallAdaptertothe
Controller.Theorangelightontheside
oftheControllerwillilluminatewhilein
charging mode.
C.
UnplugtheACWallAdapterfromthewall
outletanddisconnectitfromtheController,
afterchargingiscomplete.
*NOTE:Duringbatterycharging,itisnormal
forthecontrollertoexhibitamoderate
increaseinoperatingtemperature.Oncethe
batteryisatfullcapacity,batterycharging
automatically stops and the controller will
return to an ambient state.
*NOTE:Forbatterychargingandrecharging
incountriesoutsidetheUSAwithdifferent
power grid requirements, the plug blades
should be replaced on the charger adapter to
conformtolocalpowergridrequirements.
Battery Safety Warning
CAUTION: The Biomet®EBI Bone Healing
System control unit contains a permanently
installed Lithium Ion battery and cannot be
replaced by a service technician. You must
readandfollowthesesafetyinstructionsand
warningsinconjunctionwiththeImportant
safeguardsprovidedwithinthismanualbefore
using or charging the battery within your
control unit.
•Donotattempttoopenthecontrolunitto
access the battery or its internal electronic
componentsforanyreason.Nounauthorized
modificationofthecontrolunitisallowed.
•Neverattempttochange,adjustorreversethe
battery’spolarityconnectionsforanyreason.
•Donotalloworpermitthecontrolunitorits
permanently installed battery to be physically
mishandled, abused, crushed, mutilated or
penetratedbyanymetalobjectsuchasanail.
•Neveralloworpermitanymetalobjectto
touch or contact the permanently installed
battery’s terminals.
•Neverstorethecontrolunitorchargethe
batteryinextremetemperatures.
•Neverchargethebatteryunattended.
•
Alwayskeepthecontrolunitawayfromchildren.
Anyabuseormisusetothecontrolunitorits
permanently installed battery may result in seri-
ouspersonalinjuryand/orpropertydamage.
Biomet®isnotliableforanysuchabuse,
misuse or resulting damage.
Toensurepropercharging,ONLYUSEtheAC
WallAdaptersuppliedwithyourdevicesystem
andfollowthespecificoperatinginstructions
provided within this manual.
Alwayskeepthecontrolunitanditsperma-
nently installed battery dry.
Whenalltreatmenthasbeencompleted,the
control unit and permanently installed battery
8
9
Battery Safety Warning (continued)
MUSTBEdisposedofproperly.IntheUSA,
disposalinformationmaybeobtainedby
contacting the Rechargeable Battery Recycling
Corporation Hotline (RBRC)at1-800-822-8837.
Pleasecontactlocalrecyclingauthoritiesfor
properdisposalinformationandinstructionsif
outsidetheUSA.
Neverdisposeinnormalhouseholdwasteorrefuse.
Normalchargingtemperaturerangeis50°F
(10°C)to+95°F(35°C).
Step 2: Preparing the System to Begin Treatment
The Biomet®EBI Bone Healing System includes
twolinkcables:“zero”lengthand28".The
cableexitingthecontrolleris41/2"long.All
SFLXTreatmentCoilshavean8"cable.Hence,
useofthe“zero”lengthlinkcablewould
separatethestimulatorcontrollerfromthe
treatmentcoilbyapproximately14".Useof
the28"linkcablewouldseparatethecontroller
andtreatmentcoilbyapproximately41".A
linkcableof48"isavailablefromBiometasa
replacementpart.(Seeorderinginformation
page24.)The48"and28"linkcablescannotbe
usedwiththeSFLX5treatmentcoil.
*NOTE: The Biomet®EBI Bone Healing System
isnotcompatiblewiththeFLX®Coilsusedwith
the EBI Bone Healing System®–Model2001.
Afterconfirmingthatthelinkcableisproperly
connected to the controller, connect the other
endofthelinkcabletotheSFLXFlexible
TreatmentCoil.Thisconnectionallowsforsimple
quick disconnect and reconnect by the patient.
Next,positiontheSFLXFlexibleTreatment
Coiloverthefracturenonunionsite.Theentire
fracturenonunionsiteshouldbecentered
withinthecoiltreatmentwindow.During
treatment,thepositionofthetreatmentcoil
mayshiftduetopatientactivity.Thetreatment
coilmustbeadjustedandcenteredasrequired
tocoverthefracturenonunionsite.
Step 3: Treating and Charging
Patients may treat with the system while
rechargingthebattery.Whenthepatienttreats
and charges at the same time, the treatment
time and charging message will be displayed.
To treat and charge, patients should:
1. Turn the control unit on.
2.FollowinstructionsStep1,(Page8)-
Controller
*NOTE:Iftreatingwhilethecontrollerunitis
connectedtotheACAdapter,thedisplaywill
read,“TREATING00:00BATTERYCHARGING”.
Oncethepatienthascompletedthetreatment,
theyshouldturnthecontrolleroff,andleave
itconnectedtotheACAdaptertocontinue
chargingthebattery,untiltheLEDchanges
fromorangetogreen.
TREATING00:00
BATTERYCHARGING
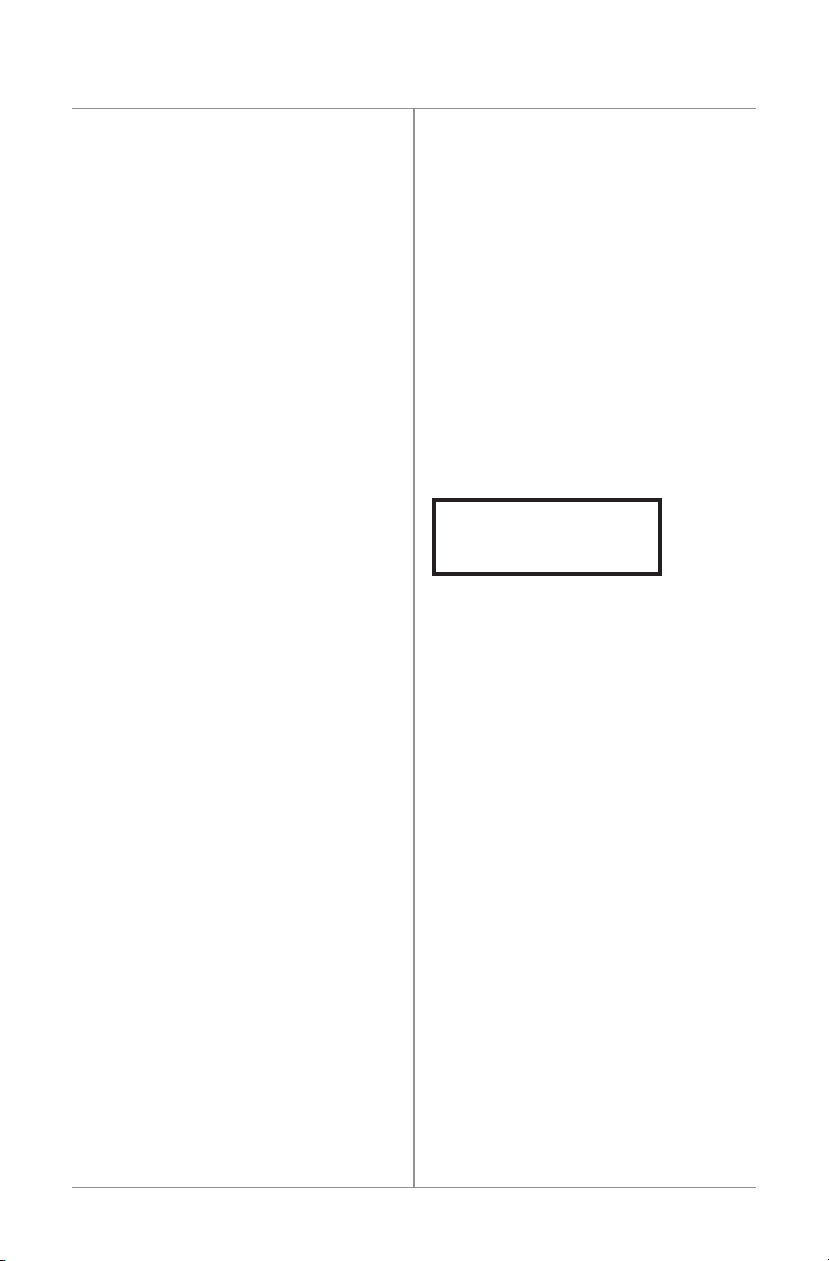
10
Operating Instructions (Continued)
Step 4: Recharging the Battery
The average daily treatment time supplied by
the battery in the controller will vary according
tothesizeofSFLXTreatmentCoilbeingused.
Thecontrollerwilldeliveraminimumof10
hoursofunsegmentedcontinuoustreatment
foralltreatmentcoilsexcepttheSFLX-4and
SFLX-5TreatmentCoils.Thecontrollerwill
deliver a minimum seven hour unsegmented
continuoustreatmentwiththeSFLX-4
TreatmentCoilandathreehourminimum
unsegmented continuous treatment with the
SFLX-5TreatmentCoil.Atroomtemperatures,
(24°C[75°F])chargingmaytakeuptothree
andone-halfhours.
Aftercompletingadailytreatmentsession,
patientsshoulddothefollowing:
A.Turnthecontrolleroff.
B.FollowinstructionsA-Cfrom
Step1.Controller(Page8)
C.Itisnotnecessarytodisconnectthe
controllerfromtheACAdapterchargeronce
fullycharged.Thecontrollercanremain
pluggedintotheACWallAdapteruntilthe
patient’snexttreatmentsession.
*NOTE: The controller’s permanently installed
batterycannotbeovercharged.Ifthecontroller
ispluggedintotheACAdapterandthebattery
isfullycharged,thechargerwillterminatethe
rechargingprocessearly.Duringthecharging
processtheLEDadjacenttotheplugwillremain
orange and once the charging is complete
theLEDwillturngreen.Therefore,donotbe
concernedifthebatteryisinadvertentlycharged
more than once.
When the Controller Needs Recharging
1. The display will read “Recharge Battery”;
“Treatment Halted” and emit three audible
beeps.Oncebeepingstops,theController
willautomaticallyshutOFF.
Afterdailytreatment,turnthecontrollerOFF
and recharge as described in step 1: Battery
Charging.(Seepage8).
*NOTE:Anexcessivelylowbatterywillresultin
noLCDdisplay.
Ifyouareexperiencingchargingdifficulties,
pleaseverifythatallelectricalconnectionsare
established. Then try charging the controller
again.Ifyouarestillexperiencingdifficulties,
contactBiomet.Foracompletelistingof
contactinformation,refertopage29.
RECHARGEBATTERY
TREATMENTHALTED
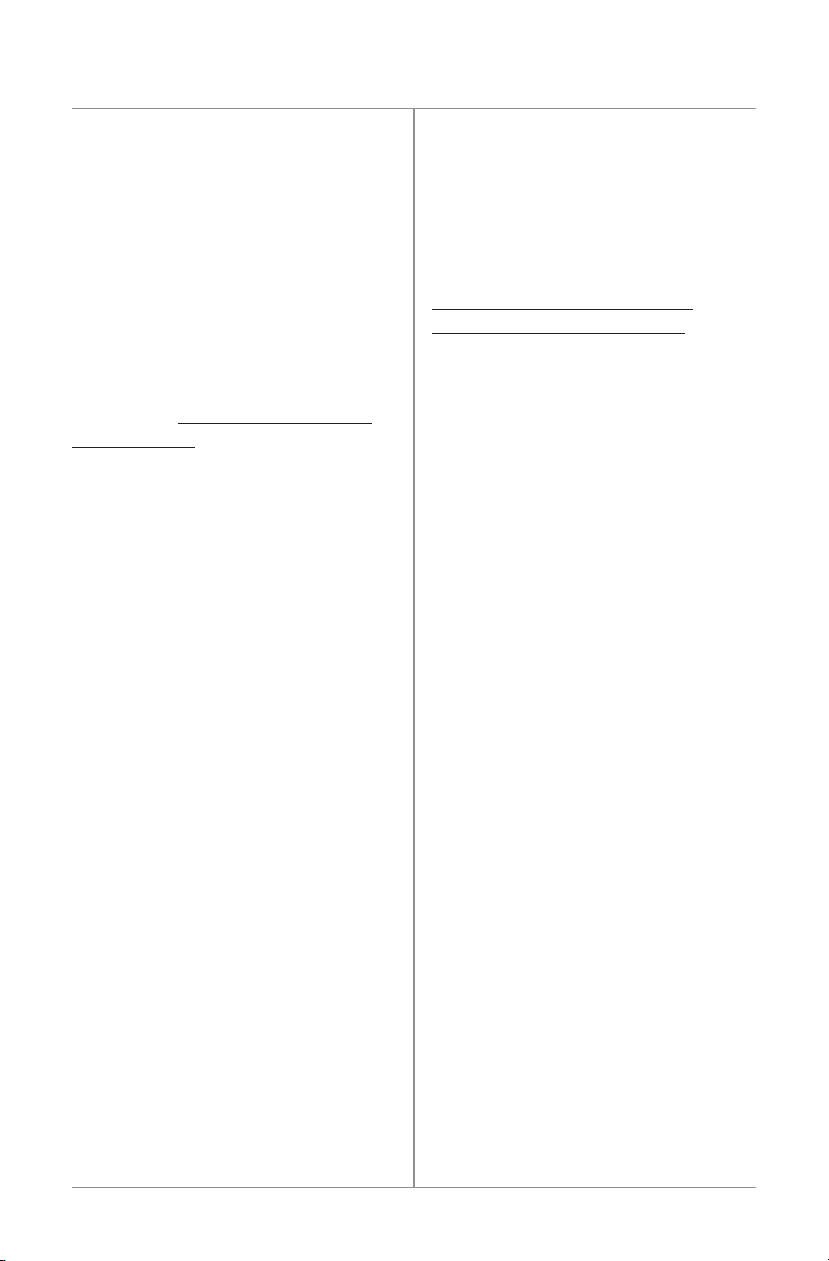
11
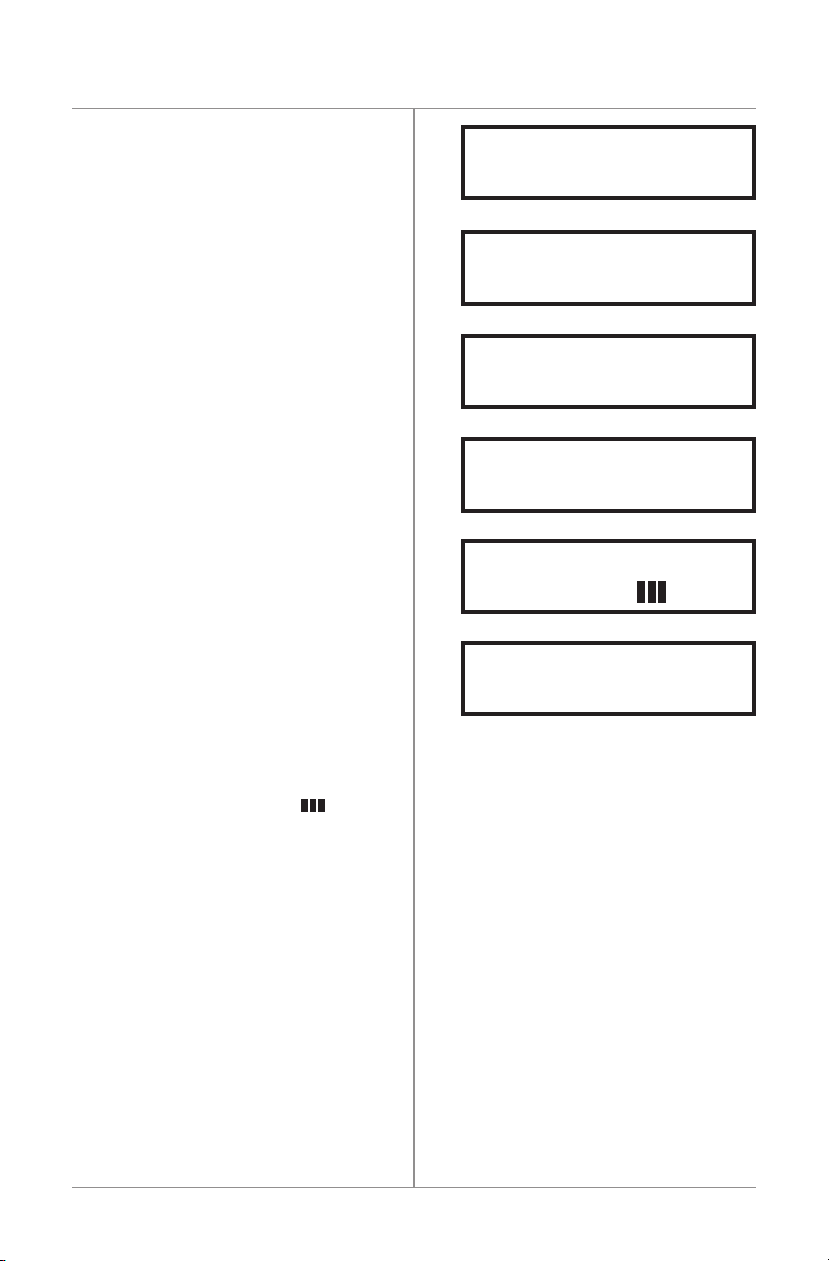
Battery Indicator Display
Thecapacityofthebatteryisdisplayedduring
thecourseoftreatment.Fourbarsindicates
afullchargewiththreebarsindicating
approximately75%charge.
California Perchlorate Label
The controller contains a permanently installed
back up battery mounted on the printed circuit
boardthatcontainsverysmallamountsof
perchlorate.Californialawrequiresthefollowing
label:“PerchlorateMaterial–specialhandling
may apply. See www.dtsc.ca.gov/hazardous-
waste/perchlorate.” There is no special handling
required by patients.
Avoid Potentially Explosive Atmospheres
Areaswithpotentiallyexplosiveatmospheres
areoften,butnotalways,postedandcan
includefuelingareas,suchasbelowdecks
onboats,fuelorchemicaltransferorstor-
agefacilities,orareaswheretheaircontains
chemicals or particles, such as grain dust, or
metal powders.
Whenthepatientisinsuchanarea,turnoff
your controller and do not charge the battery.
In such areas, sparks can occur and cause an
explosionorfire.
Controller Clip Holder
• Thecontrollermaybewornonabeltina
clip holder that is included in the system,
orintheoptionalextremityband.Theholder
will securely hold the controller during
normal treatment.
• Toinsertthecontrollerintotheclipholder,
alignthefrontofthecontrollerthatfeatures
thereset/backlightbuttonfacingawayfrom
theclipholder.Aligntheslotsonthe
controllerunitwiththepairofmatchingtabs
on the clip holder. Press the controller into
the holder until the tab engages.
Anaudibleclickshouldbeheard,thus
securing the controller to the holder.
• Adjustclipholderplacementtomaximize
patientcomfortandavoidpossibleimpacts
withfurniture,etc.
Keep the Bone Healing System and its
accessoriesawayfromsmallchildren
These devices are not toys and may be
hazardoustochildren.ForExample:
• Achokinghazardmayexistforsmall
detachable parts
• Improperusecouldresultinexposuretoa
treatmentsignalforwhichtherisksof
exposuretochildrenhavenotbeen
established
Driving Precautions
Theuseofanoninvasivebonehealingsystem
while driving may cause or contribute to
potentially hazardous distractions. Patient
shoulddiscontinueusewhiledrivingifthis
occurs.
12
Operating Instructions (Continued)
Keypad Functions
On/Off Button
EachtimetheON/OFFbuttonispressed,
an audible beep will be heard. To turn the
controlleron,presstheon/offbuttononetime.
Pressingthisbuttonasecondtimeforatleast
onesecondwillturnthecontrolleroff.Every
time the controller is turned on, the display will
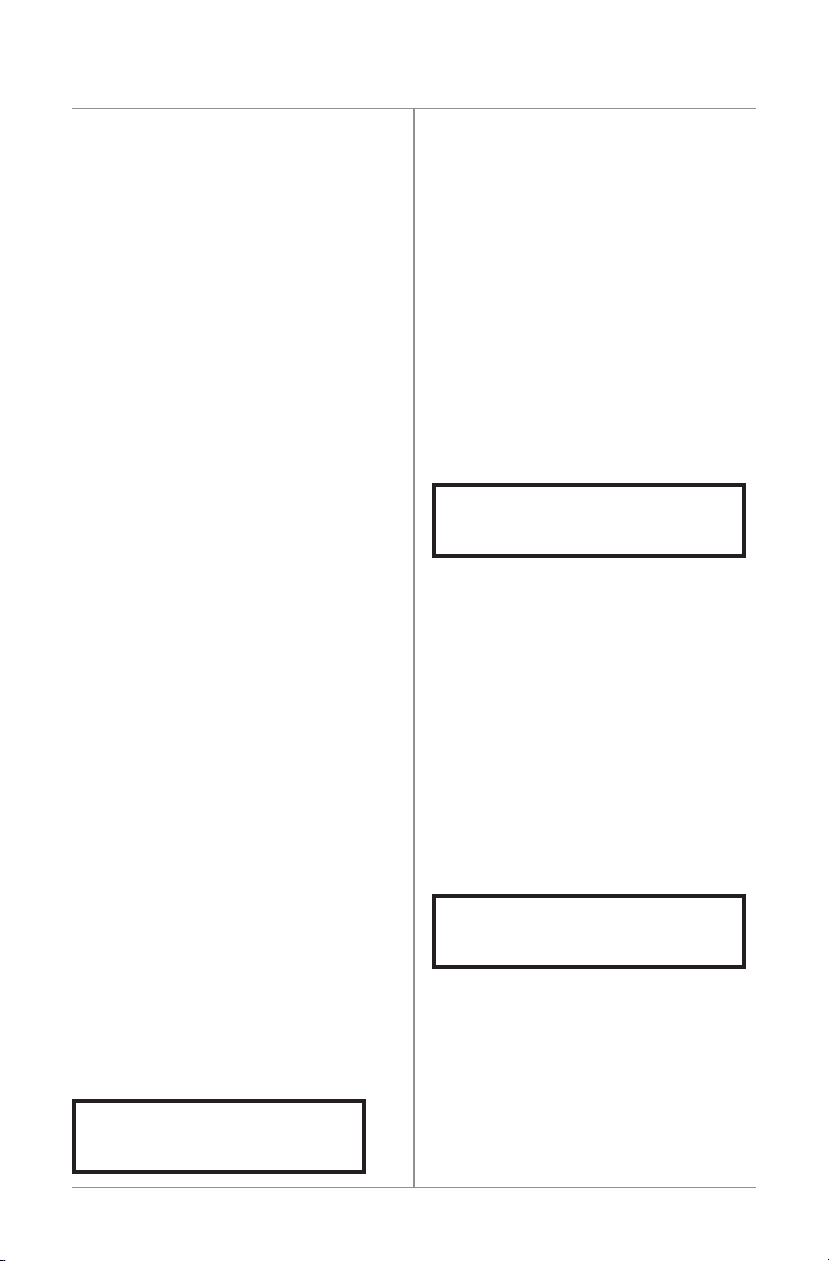
indicatethefollowingsequence:
1.“RECOMMENDEDUSE10HOURSPER
DAY”fortheBiomet®EBI Bone
Healing System.
2.“PATIENTUSAGE”;“AVGHR/DAY00:00”-
This is the daily treatment average since the
patient’s treatment started.
3.“PATIENTUSAGE”;“DAYSUSED000”-
Thisisthetotalnumberofdaysof
treatment.
4.“PATIENTUSAGE”;“DAYSUNUSED000”-
Thisisthetotalnumberofdayswhenthere
was no treatment.
5.“TREATING00:00”;“BATTERY ”-
Thisisthecumulativenumberofhours
oftreatmentinthepresentorprevious
treatment session, provided that the reset
button has not been pressed (see RESET
BUTTON).
6.PressingtheRESETbuttonwillresultin
thedisplayreading“RESETTING...”forap-
proximatelythreeseconds.Thedisplaywill
then return to display as indicated in number
fiveabove.
1.
2.
3.
4.
5.
6.
RECOMMENDEDUSE
10HOURSPERDAY
PATIENTUSAGE
AVGHR/DAY00:00
PATIENTUSAGE
DAYSUSED000
PATIENTUSAGE
DAYSUNUSED000
TREATING0:00
BATTERY
RESETTING...
RESET and Display Backlight Button – Located
on the Body of the Controller
The controller is designed with a backlight
functiontoenhancethevisibilityoftheLCD
displayindimlighting.Whenpressedbriefly,
thedisplay’sbacklightwillturnonfor5
seconds. The system is designed with a reset
functiontoallowthedailytimertobereset
tozero.Shouldthepatientnotfinishtheten
hoursoftreatmentinoneday,he/shepresses
theRESET/Backlightbuttonfortwo(2)beeps
and the daily timer will go back to zero in
preparationforthenexttreatmentsession.The
treatment time will be retained on the display
unlesstheRESETbuttonisdepressedfortwo
audible beeps.
13
Toavoidtheaccidentalresetofthedailytime,the
RESETfunctionhasathreeseconddelay.When
pressedformorethanonesecond,thecontroller
will beep. To clear the time back to zero, continue
holding the button until a second beep is heard
(approximatelythreeseconds).Releasethe
RESET/Backlightbutton.Thiswillclearthetime
backto0:00.
Followingcompletionofyourdailytreatment,
youshoulddothefollowing:
1.Makesurethecontrollerisoff.Ifyou
havecompleted10hours,theunitwill
automaticallyshutoff.Withlessthan10
hoursoftreatment,youwillneedtomanually
turnthecontrolleroff.
2.InserttheACAdapterchargerintothe
controllertorechargethebatteryforyour
nexttreatment(seeStep1:BATTERY
CHARGINGpage8).
The Biomet®EBI Bone Healing System may
be used at home or at work. Your patient’s
scheduleandlifestylewilldeterminethebest
timeforusingthesystem.Manypeoplefindit
convenient to treat while they are sleeping.
Troubleshooting System Messages
Allowuptooneminuteforthedisplaymessage
tochange,aftertakingcorrectiveaction.
• “RECHARGE BATTERY TREATMENT
HALTED”Ifthismessageappears,the
battery needs to be recharged. This message
will only appear during a treatment session.
In order to recharge the battery and
continuetreatment,connecttheACWall
Adapterandturnthecontrolleron.Refer
toStep4:(Page10)“RECHARGINGTHE
BATTERY”
“Check Connectors See Manual”
This message, accompanied by audible beeps,
appears when the controller is not properly
connectedtotheSFLXTreatmentCoilwhen
the controller is turned on. The controller will
turnitselfoff.Besuretocheckallelectrical
connections between the controller, link cable
andtreatmentcoil.Whenthecontrolleris
turned back on and the message continues,
check all cable connections again. Ifyouneed
assistance,youshouldcall1.973.299.9300
and ask to speak to a Patient Support
Representative.OutsidetheUnitedStates
contactyoulocalEBI/BiometDistributor.
”Check Coil See Manual”
This message, accompanied by three to
fouraudiblebeeps,appearswhentheSFLX
TreatmentCoilisdisconnected,damaged,
orinappropriatelyflexed.Themessagewill
stay on the display until the controller turns
itselfoff.Ifyouneedassistance,youshould
call1.973.299.9300andasktospeaktoa
PatientSupportRepresentative.Outsidethe
UnitedStatescontactyourlocalEBI/Biomet
Distributor.
RECHARGEBATTERY
TREATMENTHALTED
CHECKCOIL
SEEMANUAL
CHECKCONNECTORS
SEEMANUAL
14
Operating Instructions (Continued)
“Cannot Treat Call Biomet 1.973.299.9300”
This message, accompanied by three audible
beeps, appears when there is a hardware
problem within the controller. The message
“CANNOTTREAT”willappearandalternate
with“CALLBIOMET1-973-299-9300”.If
the patient needs assistance, they should
call1.973.299.9300andasktospeaktoa
PatientSupportRepresentative.Outsidethe
UnitedStatescontactyourlocalEBI/Biomet
Distributor.
“System Endpoint Call Biomet”,
Whenthecontroller’sinternaltimerreaches
400daysofoperationfromthefirstonehour
treatmentor270therapeutictreatments,the
treatmentsignalislockedoff.Thecontroller’s
LCDdisplaywillread“SYSTEMENDPOINT
CALLBIOMET”Thismessagewillappear
everytimethecontrolleristurnedon.Atthis
point, the system should be discarded. It is
recommended that the patient contact the
prescribing physician indicating that they have
reached this point in their treatment. See page
22forDisposalInstructions.
CANNOTTREAT
CALLBIOMET
1-973-299-9300
Alternates with
SYSTEMENDPOINT
CALLBIOMET
Compliance Data Software Introduction
(Physician Use Only)
This bone healing system contains embedded
softwareandfirmwarewhichallowsthedisplay
ofpatient-specifichistorydataincludingusage
andtherapeutictreatmenttimeviauseof
BiometComplianceDataDownloadSoftware.
TheComplianceDataDownloadSoftwareis
classifiedasamedicaldevicedatasystem
(MDDS).Itsprimaryfunctionistheelectronic
retrieval,transfer,display,andstorageof
devicegenerated-specificpatienthistorydata,
withoutalteringthefunctionorparametersof
theconnecteddevice.Thissoftwareisonly
intendedtobeusedandinterfacewiththe
followingBiometdevices:
• Biomet®EBI Bone Healing System
• Biomet®SpinalPak®Non-invasiveSpine
FusionStimulatorSystem
• Biomet®OrthoPak®Non-invasiveBone
Growth Stimulator System
Thesoftwareisavailableasadownloadable
executablefiletoBiometrepresentativesonly
through a secure web portal.
Patient Usage Reports (Physician Use Only)
TheComplianceDataDownloadSoftwarewill
downloadandtransferdevice-specificpatient
historydataviaaUSBcablefromtheattached
devicetoapersonalcomputer(PC)anddisplay
itonthePC’smonitor.Itcanthengeneratea
read-onlyreport,inAdobePDFformat,which
can be saved or printed. The device does
not record a patient name or diagnosis. The
analysisofthedownloadedpatienthistorydata
must be limited to the prescribing physician.
Theprintout/displayincludes,amongother

15
information,daysused,averagehoursperday,
daysun-usedanddayslapsed.Totaltreatment
timeisdisplayedalongwithagraphofthe
numberoftreatmentsessionsrecordedat
specifictreatmentdurationhourintervals.
Careshouldbetakennottomanipulatedatain
any way.
Biomet recommends patients bring their
controllertoeachfollow-upvisitsothat
accumulated compliance data can be accessed,
downloaded and reviewed by their prescribing
physician at that time.
In the event patient data reveals a compliance
deviation with the prescribed treatment
regimen, the prescribing physician may discuss
treatment options or alternatives including
an evaluation regarding ongoing compliance
in the patient’s overall prescribed therapeutic
treatment regimen.
Set-Up (Physician Use Only)
Beforestarting:
1)ObtainaUSBcableforcompliancedownload
forpatienthistorydatatransfer(yourBiomet
representative has this).
2)Call/ContactyourlocalBiometrepresentative
toarrangedownloadoftheComplianceData
DownloadSoftware.
ConnecttheUSBAccessorycablefor
compliancedownload(PN1067725-00)to
thedevice’sUSBportlocatedonthesideof
thecontrollertotheleftofthepowerbutton.
ConnecttheUSBcabletoanopenUSBporton
aPC.Turnonthedevice.OpentheCompliance
DataDownloadSoftwareonthePC,andan
introductoryscreenwillappear.Followthe
instructions.
Troubleshooting
Ifconnectionorprintingproblemsoccur,
please check all connection cables or your
network administrator. Please call Biomet (page
29)shouldanyadditionalproblemsarise.
Warnings
TheComplianceDataDownloadSoftwaremust
only be run on a personal computer operated
byorundertheguidanceandsupervisionofa
Biometsalesrepresentative.Thissoftware
isonlyintendedtobeusedwiththefollowing
Biomet devices:
• Biomet®EBI Bone Healing System
• Biomet®SpinalPak®Non-invasiveSpine
FusionStimulator
•Biomet®OrthoPak®Non-invasiveBone
Growth Stimulator System
16
Operating Instructions (Continued)
Patient Usage Report
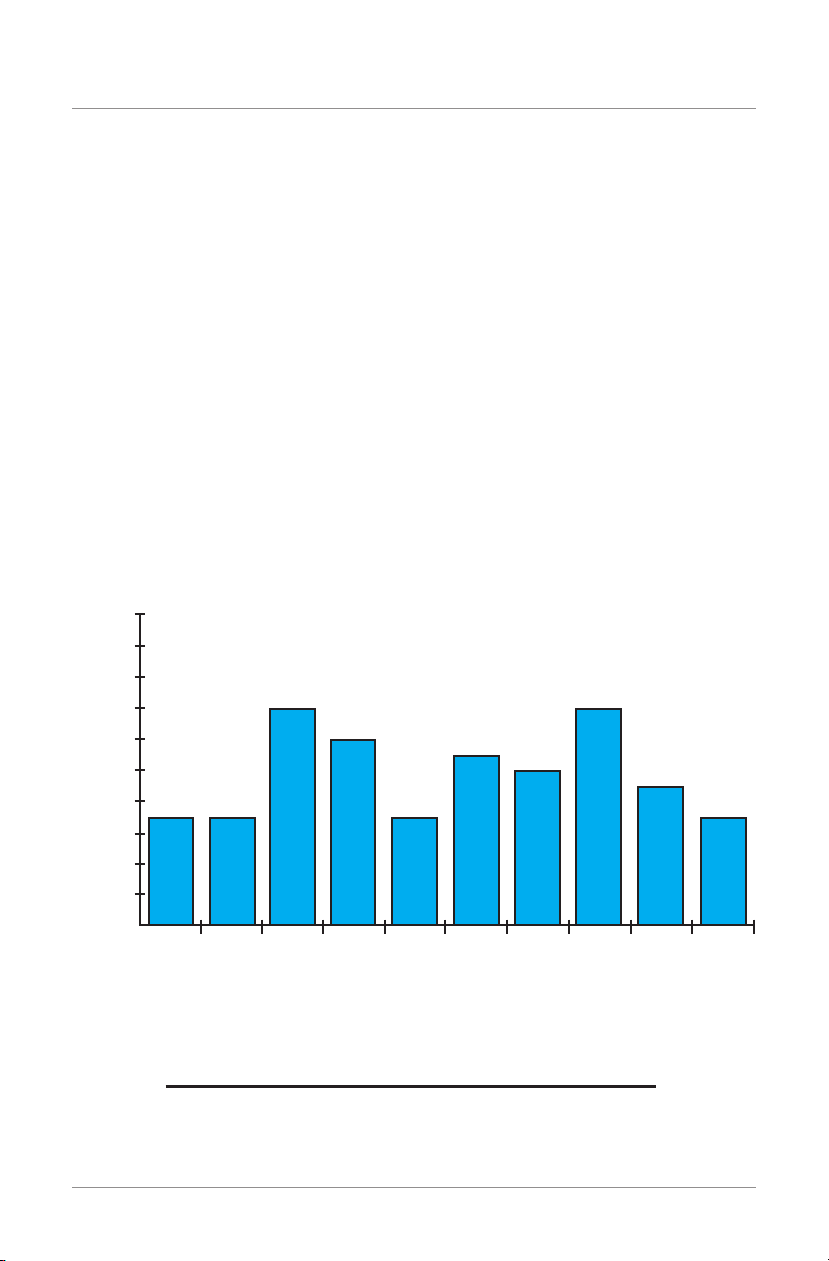
Device: Biomet®EBI Bone Healing System
Serial Number: 0002 Days Lapsed: 1
Days Used: 78 Days Unused: 21
Avg Hours / Day: 4:13 Avg Hours / Session: 6:22
Total Treatment Time: 417 Hours Treatment Period: 123
Days
Demonstration mode – Invalid Data
Frequency of Treatment Dose
20
18
16
14
12
10
8
6
4
2
00-1 1-2 2-3 3-4 4-5 5-6 6-7
7777
14 14
12 11 10 9
7-8 8-9 9-10
Treatment Hours
NumberOfSessions
Sample
Patient Usage Report
Device: Biomet®EBI Bone Healing System
Demonstration mode – Invalid Data
17
SFLX Treatment Coils
Depth of Penetration Specifications for SFLXTreatment Coils
SFLX 1, 2, 3, 4, 5, and Coilette Treatment Coils Tolerances
SFLXCoil M/L Flexion Depth of Vertical Anatomical
Penetration
Fracture Length
Location(s)
SFLXMiniCoilette Minimum 2.5cm 2.5cm 1.5cm
Maximum 3.5cm 2cm 1.5cm
SFLXCoilette Flat N/A 2.75cm 4cm
Elliptical N/A 3.5cm 4cm
Saddle N/A 3.5cm 2cm
SFLXXL Flat N/A 3.5cm 6cm
Coilette Elliptical 5cm 4.25cm 4cm
Saddle 7.5cm 5.5cm 4cm
SFLX1 Minimum 9cm 6.5cm 7cm
Maximum 13cm 5.5cm 6cm
SFLX2 Minimum 8cm 8cm 10cm
Maximum 11cm 7cm 10cm
SFLX2-1 Minimum 10cm 5cm 16cm
(Elliptical) Maximum 12cm 4.5cm 14cm
SFLX 2-1 Minimum 8cm 8cm 10cm
(Saddle) Maximum 11cm 7cm 10cm
SFLX2-4 Minimum 8cm 8cm 10cm
Maximum 11cm 7cm 10cm
SFLX3 Minimum 5cm 7cm 7cm
Maximum 9cm 5.5cm 6cm
SFLX4 Minimum 9.25cm 10cm 12cm
Maximum 14.5cm 8cm 8cm
SFLX4-1 Minimum 9.25cm 10cm 12cm
(Saddle) Maximum 14.5cm 8cm 8cm
SFLX4-1 Minimum 12cm 6cm 22cm
(Elliptical) Maximum 14cm 6cm 18cm
SFLX4-4 Minimum 9.25cm 10cm 12cm
Maximum 14.5cm 8cm 8cm
SFLX5 Minimum 13cm 12cm 10cm
Maximum 20cm 10cm 10cm
phalanges
clavicle, metatarsals,
scaphoid, distal
radius, cuboid, medial
lateral malleolus
foot,hand,small
bones
metatarsals, scaphoid,
metacarpals, radius,
ulna
humerus,tibia,fibula,
radius, ulna
tibia,fibula,radius,
ulna, humerus
tibia,fibula,radius,
ulna, humerus
ankle
radius, ulna, metatar-
sals,distaltibia/fibula
midshaftfemur,tibia,
fibula,humerus
tibia,fibula,humerus,
radius, ulna
tibia,fibula,humerus,
radius, ulna
ankle
femur(proximalor
midshaft)
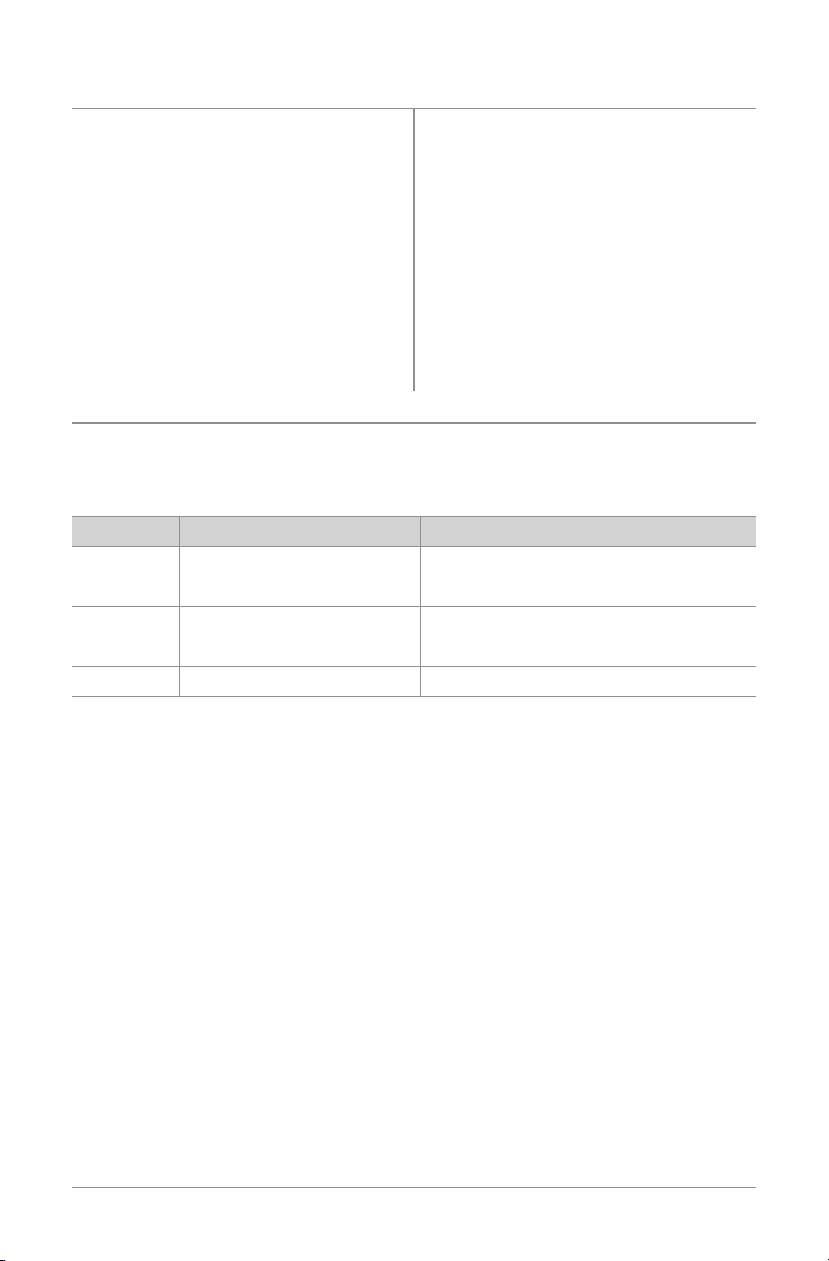
18
*NOTE: The Biomet®EBI Bone Healing
System does not include a treatment coil. The
prescribing physician and Biomet representative
willselectthetreatmentcoilsuitableforthe
particularpartofthepatient’sanatomytobe
treated.Ifinitiallytreatingwithacasttreatment
coil can be interchanged in the event a cast is
removed.ContactyourBiometRepresentative
forasuitablereplacement.The48"and28"
linkcablescannotbeusedwiththeSFLX-5
TreatmentCoil.
AllSFLXCoil–Anatomicalplacement
instructionsandFlexionGaugesareincluded
with the coil.
Specialtytreatmentcoilsareforapplications
where the standard straps may not be adequate
to secure the treatment coil. Each specialty
SFLXTreatmentCoilfeaturesone(1)snap
atallfourcornersofthecoilandcomes
pre-assembledtofittherightsideofthebody,
but the same snaps and straps may be easily
switchedforleftsideapplications.
Application Instructions for the SFLX-1, SFLX-2, SFLX-3, SFLX-4 and SFLX-5 Treatment Coils
Appliesto:
Description Treatment Coil # Suggested Placement*
SFLX-1 1068225
Metatarsals,Radius,Ulna,Scaphoid,Metacarpals
SFLX-2 1068226 Humerus,Tibia,Fibula,Radius,Ulna
SFLX-3 1068229 Radius,Ulna,Metatarsals,DistalTibia/Fibula
SFLX-4 1068235 MidshaftFemur,Tibia/Fibula,Humerus
SFLX-5 1068224 Femur–ProximalorMidShaft
*NOTE:Givencertainanatomiclocations,treatmentcoilsmayhaveatendencytomigrateawayfrom
theintendedtreatmentarea.Oftenthismovementisassociatedwithpatientactivity,mobilityorthe
underlyingsurfacethetreatmentcoilrestson(skin,shirt,cast,etc.).Thetreatmentcoilstrap(s)may
beloosenedandthetreatmentcoilmustbeadjustedandcenteredasrequiredtocoverthefracture
nonunionsite.Tightenthestrapsagaintofinishthecorrection.
*Anatomiclocationsaresuggestedbasedontreatmentcoilsizeandconfiguration.Theselectionof
asuitabletreatmentcoilisindividualtoeachpatientandshouldtakeintoaccountfactorssuchas
activitylevel,presenceofacast,patientbodymassindex(BMI),etc.
SFLX Treatment Coils (Continued)
Other manuals for EBI Bone Healing System
1
Table of contents
Other BIOMET Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual