BIOTRONIK Edora 8 SR-T User manual
Other BIOTRONIK Medical Equipment manuals

BIOTRONIK
BIOTRONIK EVIA User manual
BIOTRONIK
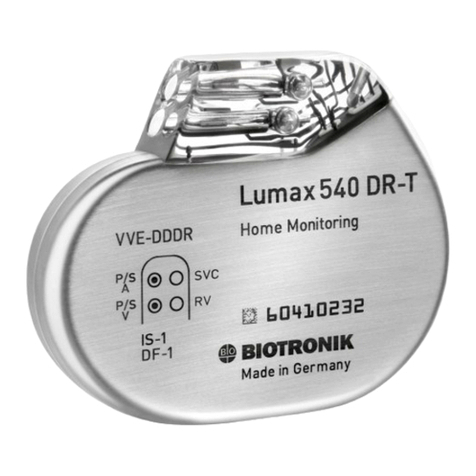
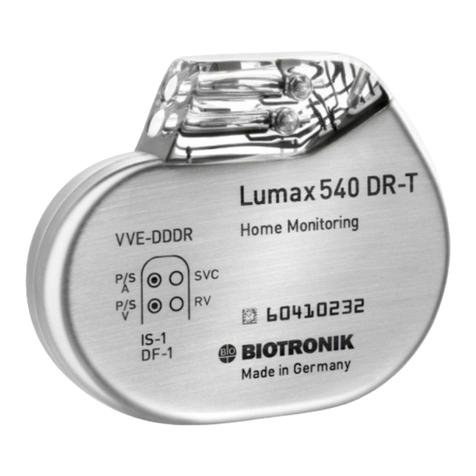
BIOTRONIK Lumax 540 VR-T DX User manual

BIOTRONIK
BIOTRONIK ICS 3000 User manual
BIOTRONIK
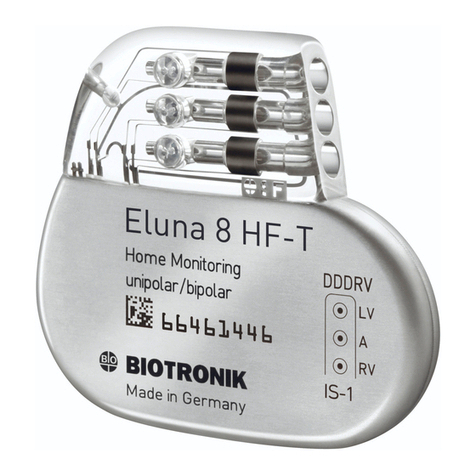
BIOTRONIK Eluna 8 User manual

BIOTRONIK
BIOTRONIK ProMRI User manual
BIOTRONIK
BIOTRONIK Pantera LEO User manual

BIOTRONIK
BIOTRONIK Enticos 4 S User manual

BIOTRONIK
BIOTRONIK ProMRI Protego DF-1 S User manual

BIOTRONIK
BIOTRONIK Amvia Stellar UDI-DI User manual

BIOTRONIK
BIOTRONIK Home Monitoring CM II-S TLine User manual

BIOTRONIK
BIOTRONIK Cardiac Airbag User manual
BIOTRONIK
BIOTRONIK Lumax VR ICD Series User manual

BIOTRONIK
BIOTRONIK Enitra 6 Series User manual

BIOTRONIK
BIOTRONIK Renamic User manual
BIOTRONIK
BIOTRONIK Pulsar-18 T3 User manual

BIOTRONIK
BIOTRONIK ProMRI Evity 6/8 User manual
BIOTRONIK
BIOTRONIK Eluna 8 User manual

BIOTRONIK
BIOTRONIK ProMRI Etrinsa 6/8 User manual

BIOTRONIK
BIOTRONIK Ilesto 5 VR-T User manual

BIOTRONIK
BIOTRONIK Lexos DR-T User manual































