CMA 0019 ∙ DK 2133 ∙ Version 001 ∙ 2022/10/25 ∙ www.customed.de
Hardware Software Hygiene
ECG ∙custo cardio 400 accu
Table of Contents
1Safety ..................................................................................................5
1.1 General notes.......................................................................................................5
1.1.1 Symbols used in this Operating Manual............................................ 5
1.1.2 Laws and regulations applicable to the product............................. 6
1.1.3 Disclaimer .................................................................................................7
1.1.4 Warranty.................................................................................................... 7
1.1.5 Support ..................................................................................................... 7
1.2 Safety installations and safe working ............................................................ 8
1.2.1 Putting into operation, setup............................................................... 8
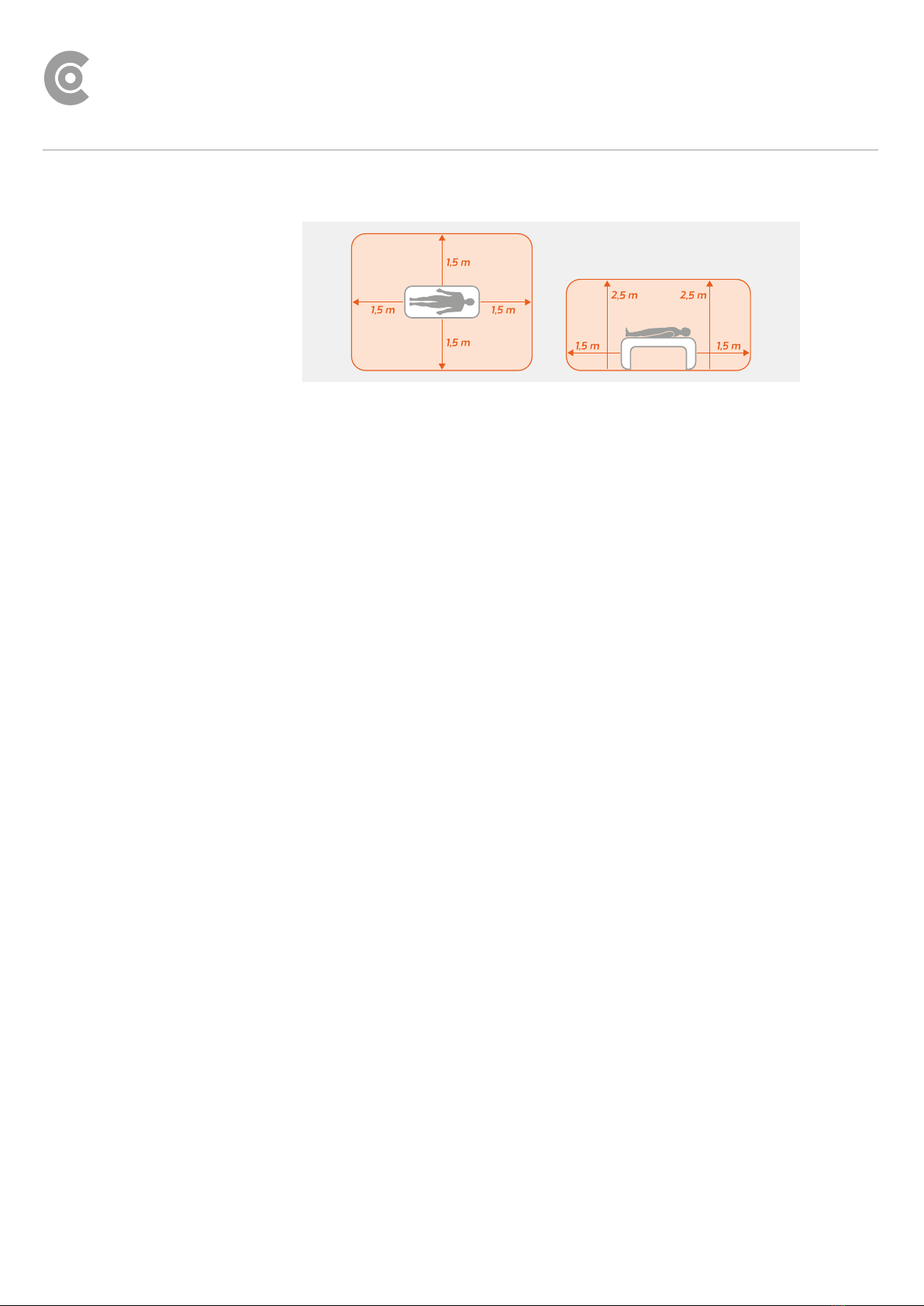
1.2.2 Ambient conditions, handling of the devices................................... 8
1.2.3 Patient safety .........................................................................................10
1.2.4 System and data security....................................................................11
1.2.5 Information on EMC (Electromagnetic Compatibility).................13
1.2.6 Maintenance (regular safety checks)................................................13
1.3 Safety instructions for resting and stress ECGs..........................................14
1.4 Residual risks resting and stress ECG...........................................................15
2Hardware...........................................................................................16
2.1 Intended use ......................................................................................................16
2.1.1 Indications and contraindications....................................................16
2.1.2 Device types and functions.................................................................17
2.2 Symbols on the devices and packaging ......................................................18
2.3 Technical data and system requirements...................................................19
2.4 Putting out of operation, storage, transport, disposal.............................22
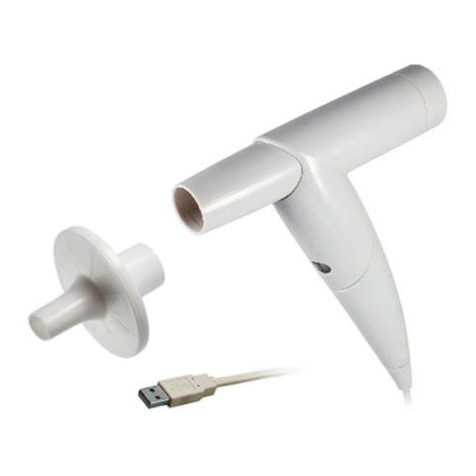
2.5 Components for the recording ......................................................................23
2.6 Mounting, preparing the device ....................................................................24
2.6.1 Getting to know the operating elements on the custo move
extension arm ........................................................................................24
2.6.2 Mounting the custo move extension arm........................................25
2.6.3 Operating the custo move extension arm.......................................28
2.6.4 Removing the custo move extension arm, disassembly..............29
2.6.5 Attaching electrode suction tubes to the device...........................30
2.7 Device operation...............................................................................................31
2.7.1 Charging custo cardio 400 accu ........................................................31
2.7.2 Restoring shipping mode....................................................................32
2.7.3 Safety mode ...........................................................................................33
2.7.4 Display and control elements ............................................................33
2.7.5 States of the suction level and status display ................................34
2.7.6 Device states in standard operation.................................................36
2.7.7 Further states and special functions ................................................37
2.7.8 custo cardio 400 accu troubleshooting ...........................................39
2.8 Procedure of an examination.........................................................................41
2.9 Attaching the recorder to the patient ..........................................................43
2.9.1 Positions of the electrodes .................................................................43
2.9.2 Notes on stress with treadmill ...........................................................44
2.9.3 Safe use of treadmills...........................................................................45
3Software............................................................................................46