CMA 0025 ∙ DK 2139 ∙ Version 001 ∙ 2022/10/25 ∙ www.customed.de
Hardware Software Hygiene
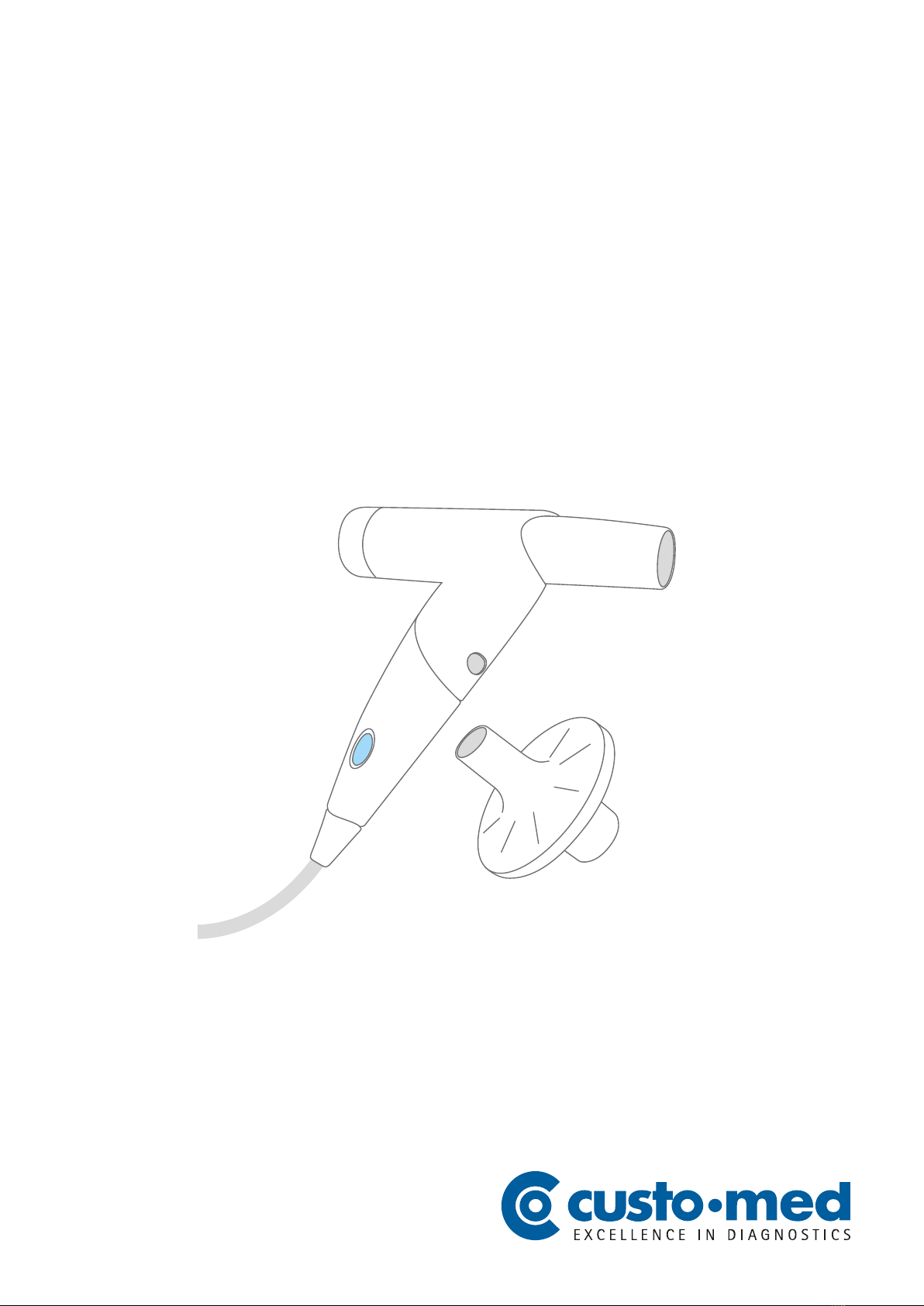
∙custo spiro mobile
Table of Contents
1Safety ..................................................................................................5
1.1 General notes.......................................................................................................5
1.1.1 Symbols used in this Operating Manual............................................ 5
1.1.2 Laws and regulations applicable to the product.............................6
1.1.3 Disclaimer .................................................................................................7
1.1.4 Warranty.................................................................................................... 7
1.1.5 Support .....................................................................................................7
1.2 Safety installations and safe working ............................................................8
1.2.1 Putting into operation, setup...............................................................8
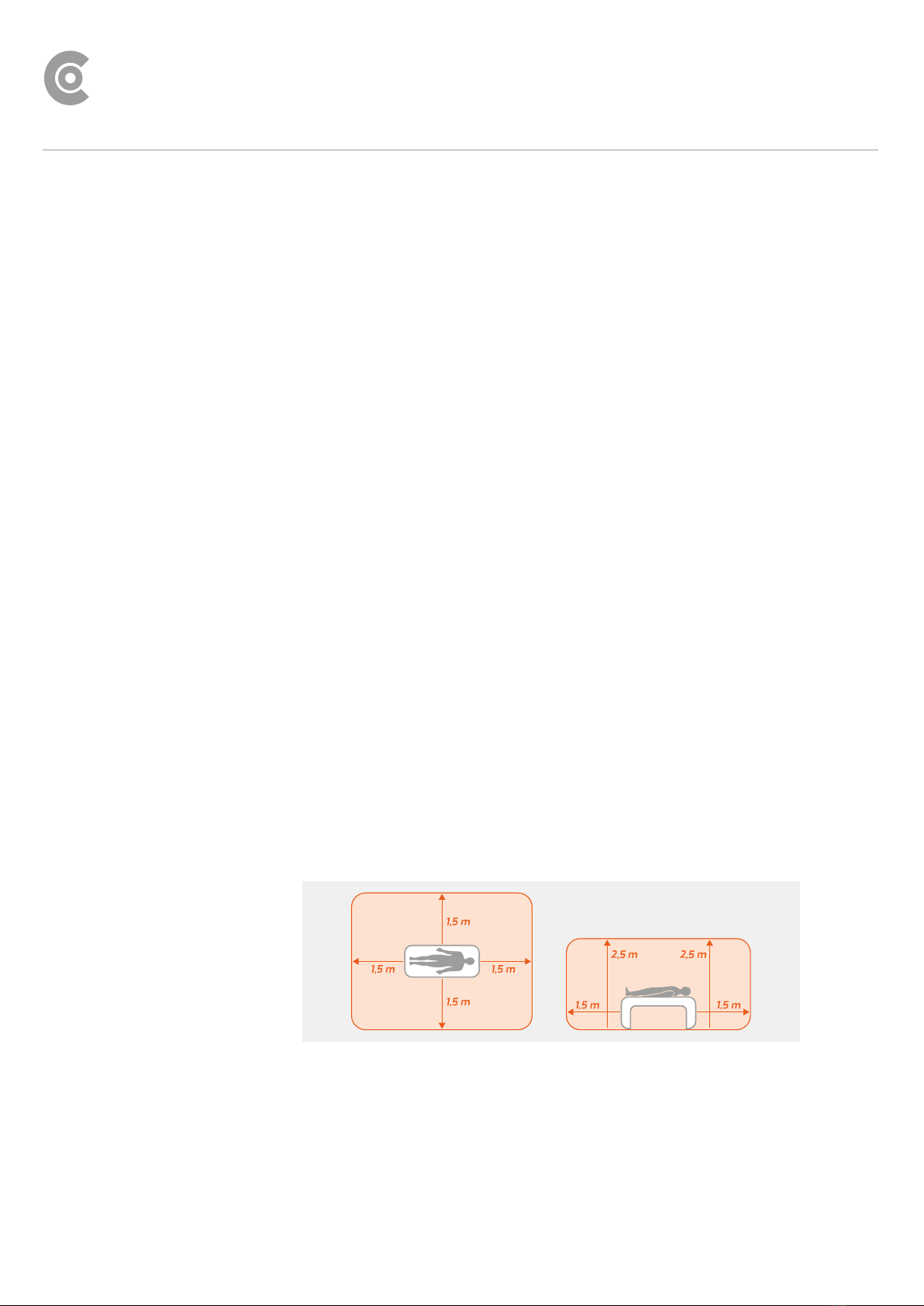
1.2.2 Ambient conditions, handling of the devices...................................8
1.2.3 Patient safety ...........................................................................................9
1.2.4 System and data security....................................................................11
1.2.5 Information on EMC (Electromagnetic Compatibility).................13
1.2.6 Maintenance (regular safety checks)................................................13
1.3 Safety instructions for spirometry.................................................................14
1.4 Residual risks spirometry................................................................................15
2Hardware...........................................................................................16
2.1 Intended use ......................................................................................................16
2.2 Symbols on the devices and packaging......................................................18
2.3 Technical data and system requirements...................................................19
2.4 Putting out of operation, storage, transport, disposal.............................22
2.5 Components for the recording ......................................................................23
2.6 Device operation...............................................................................................24
2.6.1 Function display....................................................................................24
2.6.2 Using the bacterial and viral filters ...................................................25
2.6.3 Disassembling and assembling the device.....................................26
2.6.4 Notes on calibration.............................................................................27
2.7 Procedure of an examination.........................................................................28
3Software............................................................................................29
3.1 custo diagnostic program structure.............................................................29
3.2 custo spiro mobile connection to the PC....................................................30
3.3 Calibrating custo spiro mobile ......................................................................31
3.4 Performing the spirometry measurement ..................................................32
3.4.1 Reference measurement.....................................................................32
3.4.2 Follow-up measurements: Spasmolysis and provocation..........40
3.4.3 Unconfirmed report..............................................................................42
3.4.4 Printing the measurement..................................................................44
3.5 Opening evaluations........................................................................................45
3.5.1 Opening an evaluation via the evaluation search.........................45
3.5.2 Opening an evaluation via the evaluation main menu ................47
3.6 Evaluation structure.........................................................................................48
3.7 Navigation in the evaluation ..........................................................................50
3.8 Diagnostic terms in the evaluation...............................................................51
3.9 Evaluation of the reference and spasmolysis measurement .................53
3.10 Evaluation of a provocation measurement series ....................................54
3.11 Further screens of an evaluation...................................................................55