MHW 0007 – DK 1694 | Version 002 – 15/07/2019 | custo med GmbH
2
[2] Holter hardware, custo flash 500/510 / 510V / 501/501L | page 10
Holter
Hardware, description of device for custo flash 500 / 510 / 510V / 501 / 501L
2.6 Starting the recorder and applying it to the patient
For a recording you need: a custo flash 5xx recorder, a programmed custo multiday
card (see „Part 3, Software Description“), a freshly charged battery, a clean neck
strap, a clean carrying case, three electrodes.
For multi-day recordings (additional material for the patient): three electrodes per
day, one hygiene bag per day, quick guide for patients.
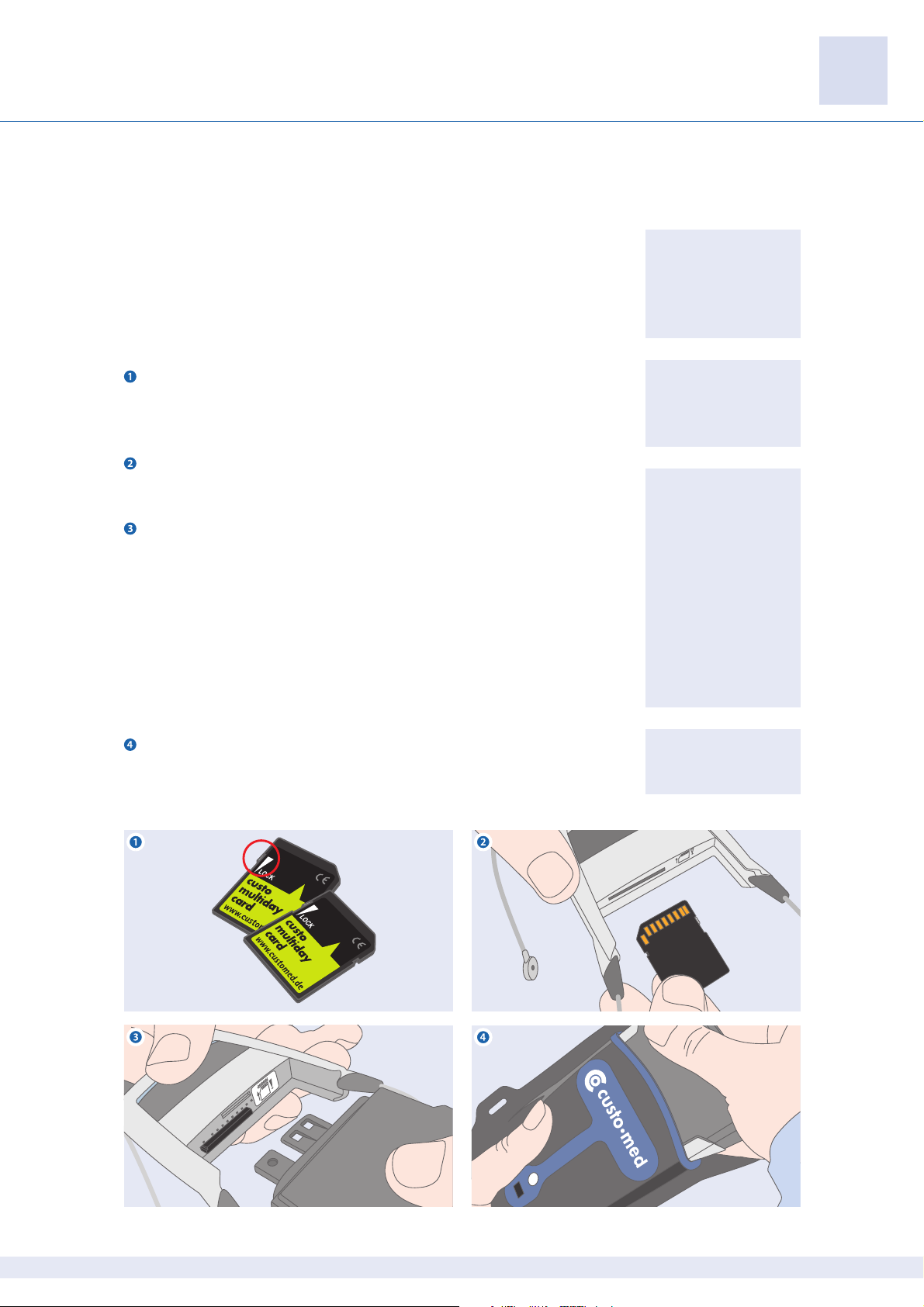
Make sure that the custo multiday card is writable. If the write protection is
active, no ECG can be saved on the SD card. To save data to the SD card, the write
protect lock must be in the upper position as shown.
Insert the programmed custo multiday card into the recorder. Make sure that
the direction of insertion is correct (see the label in the recorder).
Assemble the rechargeable battery and the recorder (press until the re-
chargeable battery engages). The direction of insertion is based on the position
of the contacts. Once the device has been put together, it takes approx. one
minute for the recording to start. During this period the custo multiday card
is configured. LED display during configuration:
2 x red. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . custo multiday card detected
green, fast. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . custo multiday card configuration
8 x red. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Configuration completed
green, slow. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Recording in progress
Put the recorder into its case. Make sure that the inspection window of the
case is on the front of the recorder and that the LED display is visible through the
inspection window.
Material for the
recording
Deactivating the
custo multiday card
write protection
Carrying case
and neck strap
Assembling and
starting the
recorder