CONTENTS
Chapter 1 - Single Wire Myograph overview........................................................................................................................................................................3
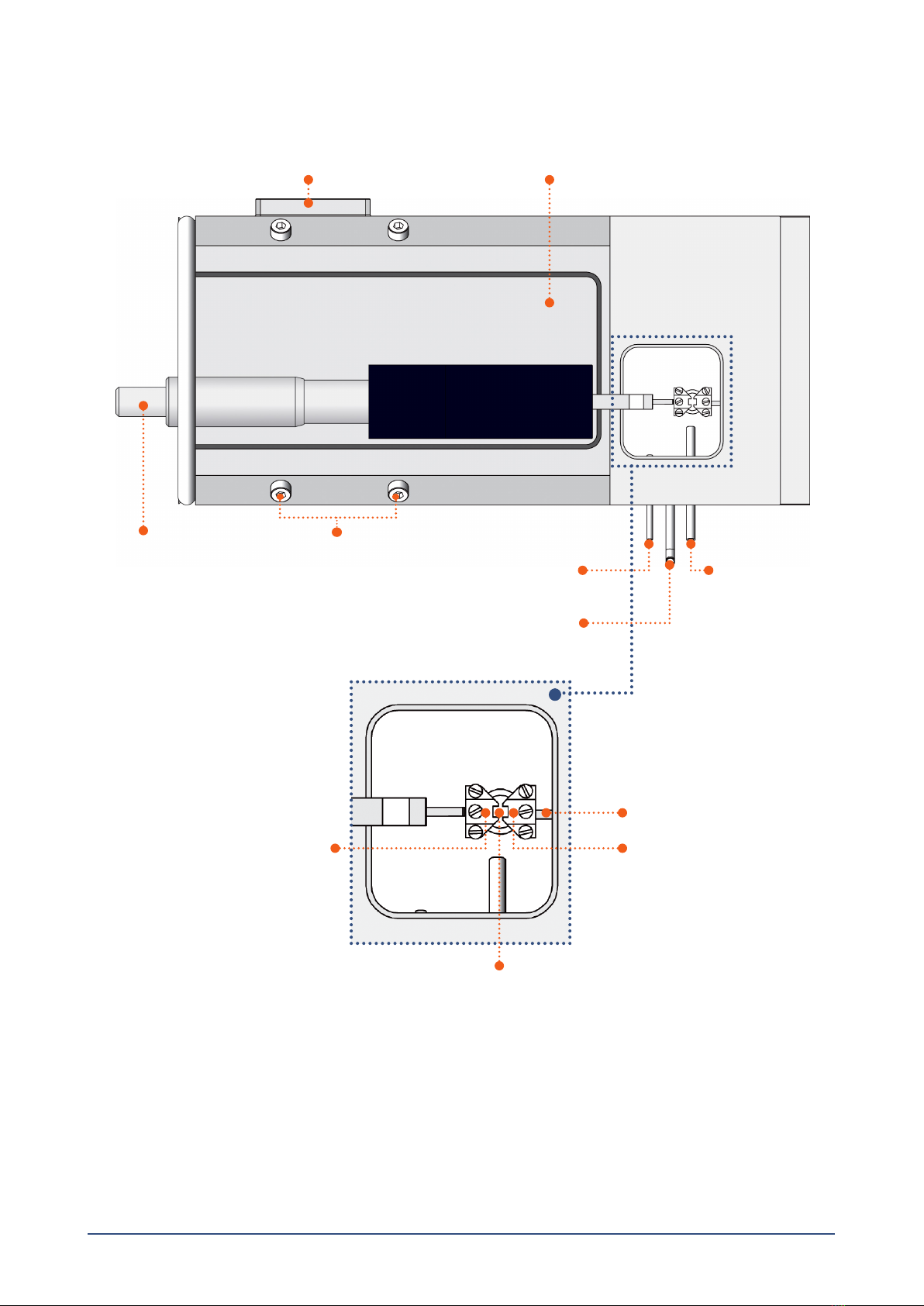
Chapter 2 - Setting up the Single Wire Myograph .............................................................................................................................................................4
2.1 Adjustment of supports.....................................................................................................................................................................4
2.2 Force transducer calibration.............................................................................................................................................................5
Chapter 3 - Experimental set-up ..............................................................................................................................................................................................6
3.1 Mounting protocol for small arteries................................................................................................................................................6
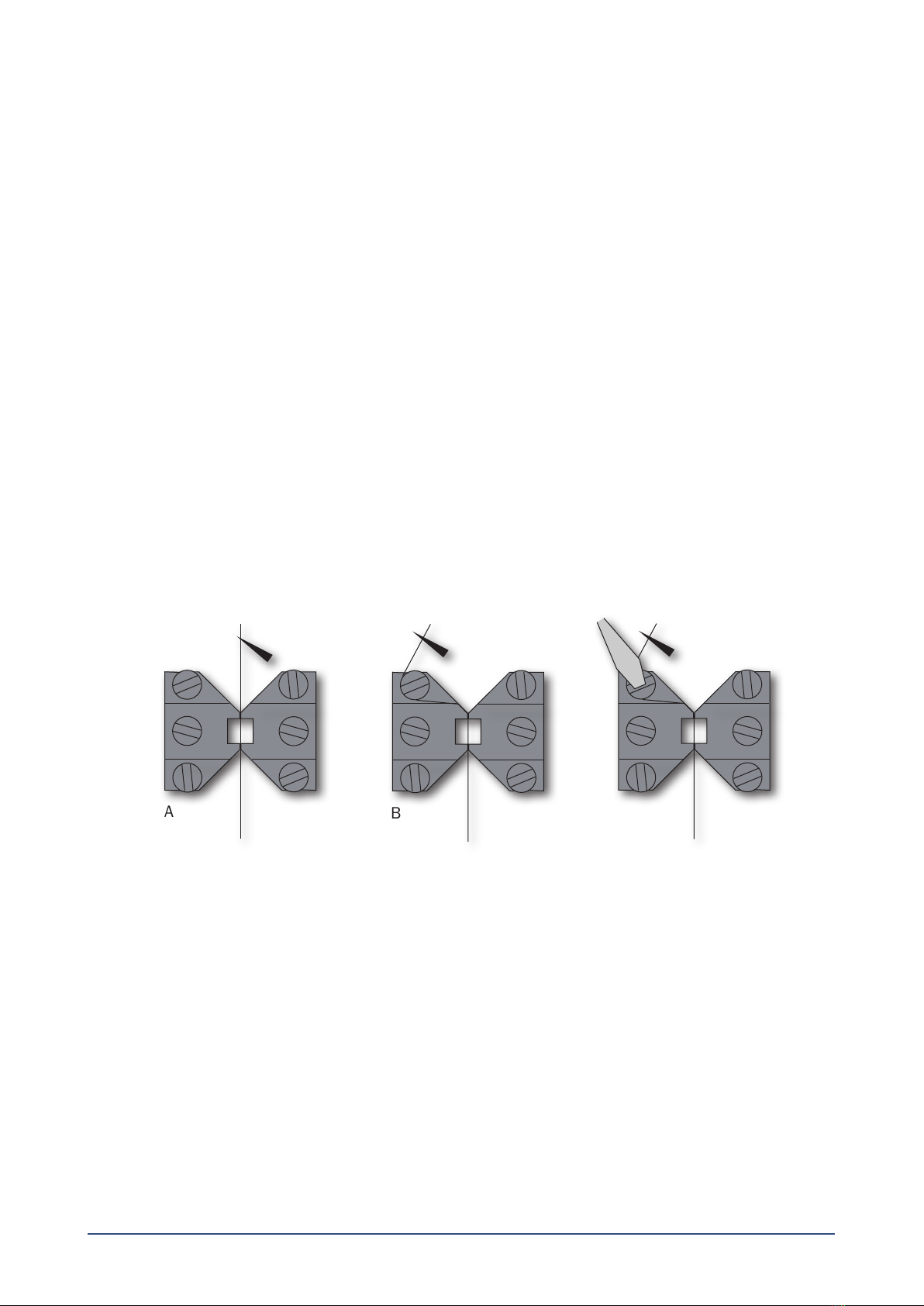
3.1.1 Mounting step one ......................................................................................................................................................................6
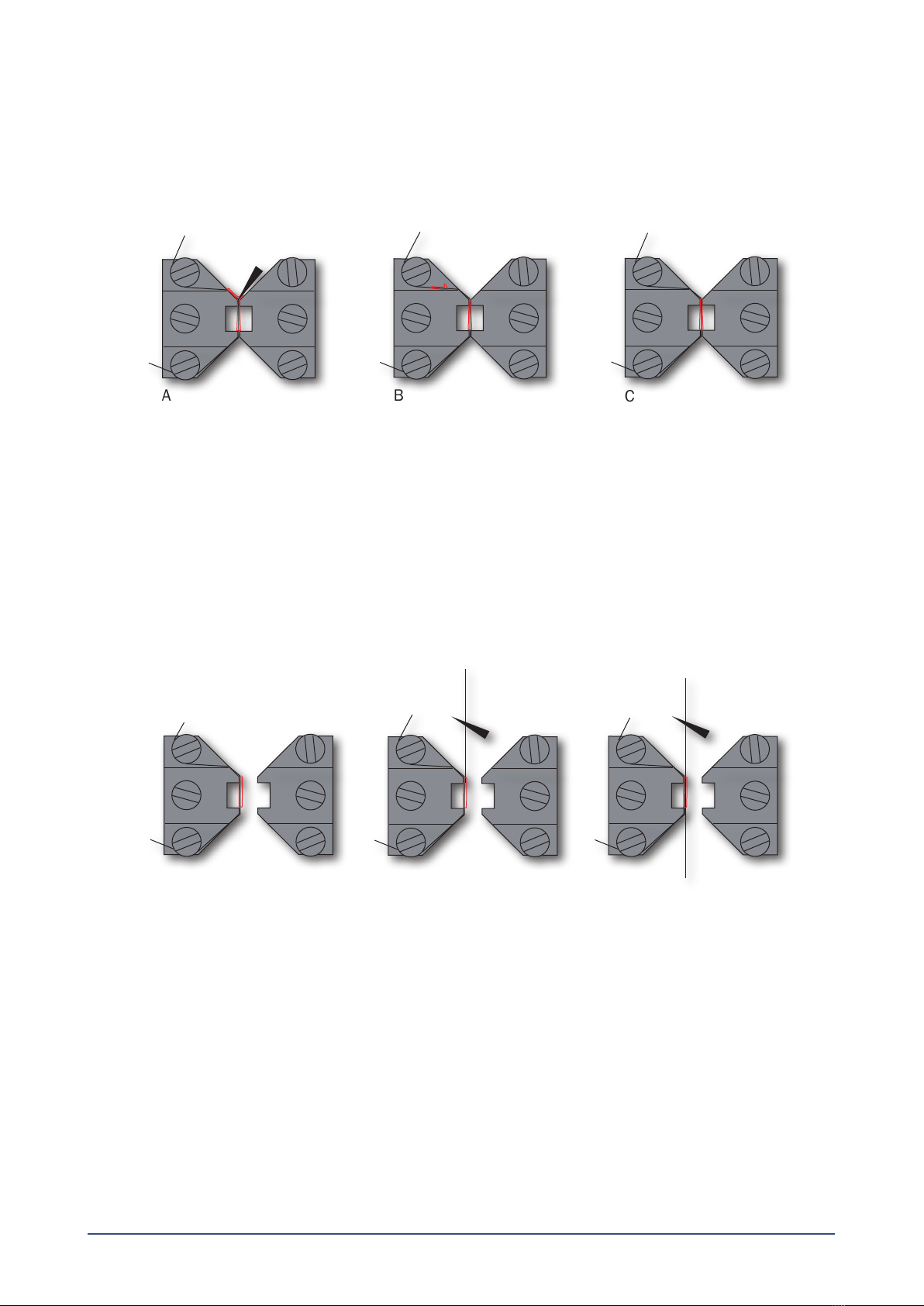
3.1.2 Mounting step two ......................................................................................................................................................................7
3.1.3 Mounting step three ...................................................................................................................................................................7
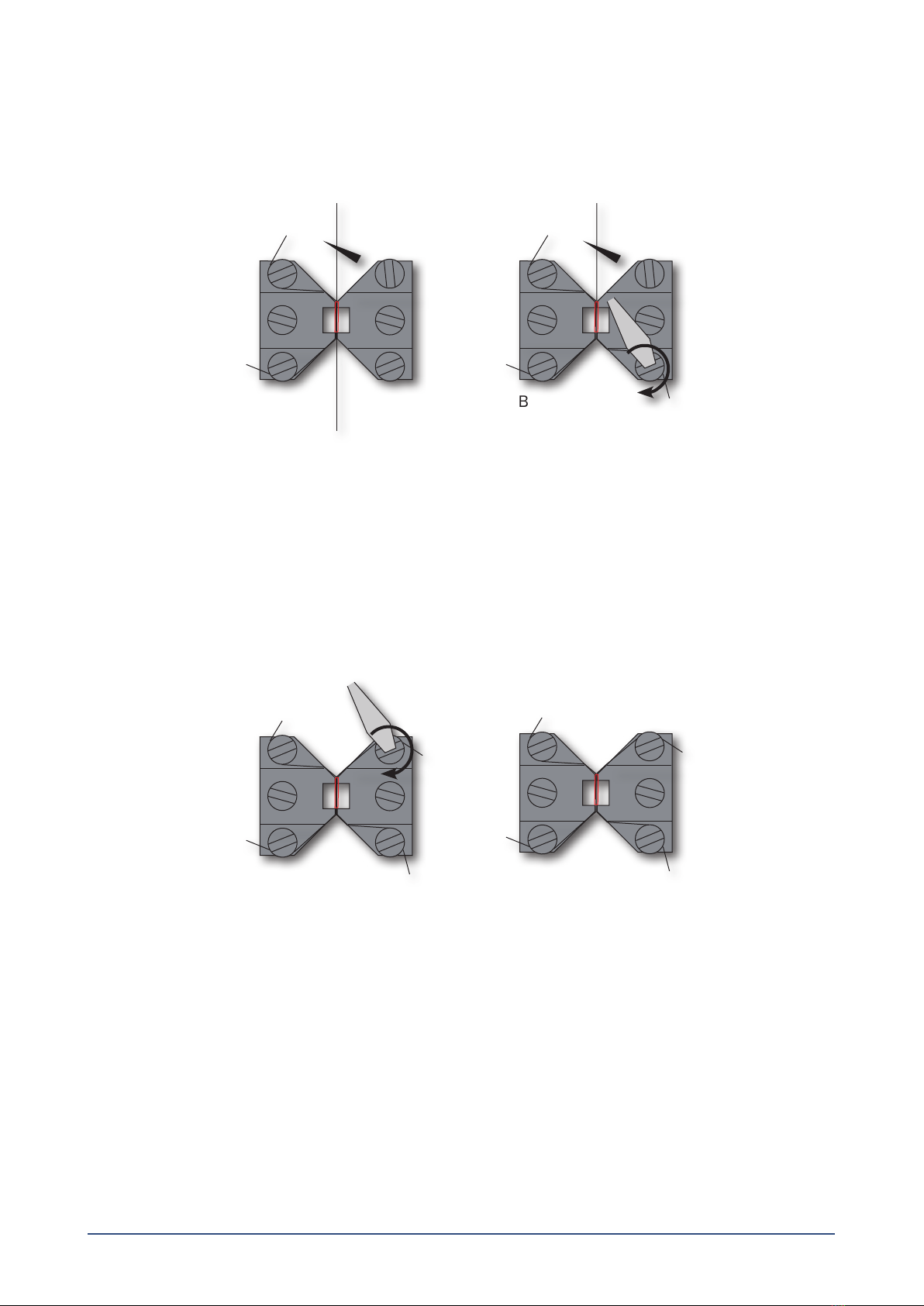
3.1.4 Mounting step four......................................................................................................................................................................8
3.1.5 Mounting step ve.......................................................................................................................................................................8
3.1.6 Mounting step six........................................................................................................................................................................9
3.1.7 Mounting step seven...................................................................................................................................................................9
3.2 Normalization ....................................................................................................................................................................................9
3.2.1 Principles of the normalization procedure ............................................................................................................................. 10
3.3 Standard start................................................................................................................................................................................. 10
3.3.1 Principles of the standard start procedure ............................................................................................................................ 10
3.4 Endothelium function..................................................................................................................................................................... 11
3.4.1 Principles of checking endothelium function......................................................................................................................... 11
3.5 In vitro experiment 1: Noradrenaline contractile response......................................................................................................... 12
3.5.1 Background .............................................................................................................................................................................. 12
3.5.2 Protocol..................................................................................................................................................................................... 12
3.6 In vitro experiment 2: Acetylcholine relaxation curve .................................................................................................................. 13
3.6.1 Background .............................................................................................................................................................................. 13
3.6.2 Protocol..................................................................................................................................................................................... 13
Chapter 4 - Cleaning and Maintenance .............................................................................................................................................................................. 14
4.1 Cleaning the Single Wire Myograph .............................................................................................................................................. 14
4.2 Maintenance of the force transducer ........................................................................................................................................... 15
4.2.1 Checking force transducer ...................................................................................................................................................... 15
4.2.2 Force transducer replacement................................................................................................................................................ 16
4.4 Maintenhance of the linear slide .................................................................................................................................................. 17
4.3 Changing the Single Wire Myograph window glass...................................................................................................................... 17
Appendix 1 - Buer recipes ..................................................................................................................................................................................................... 18
Appendix 2 - Normalization theory ...................................................................................................................................................................................... 20
Appendix 3 - Reading a millimetre micrometer............................................................................................................................................................... 22
Notes................................................................................................................................................................................................................................................ 23