3
CONTENTS
Chapter 1 - Wire Myograph overview .................................................................................................................................................................................... 3
Chapter 2 - setting up the wire myograph - 620M............................................................................................................................................................ 4
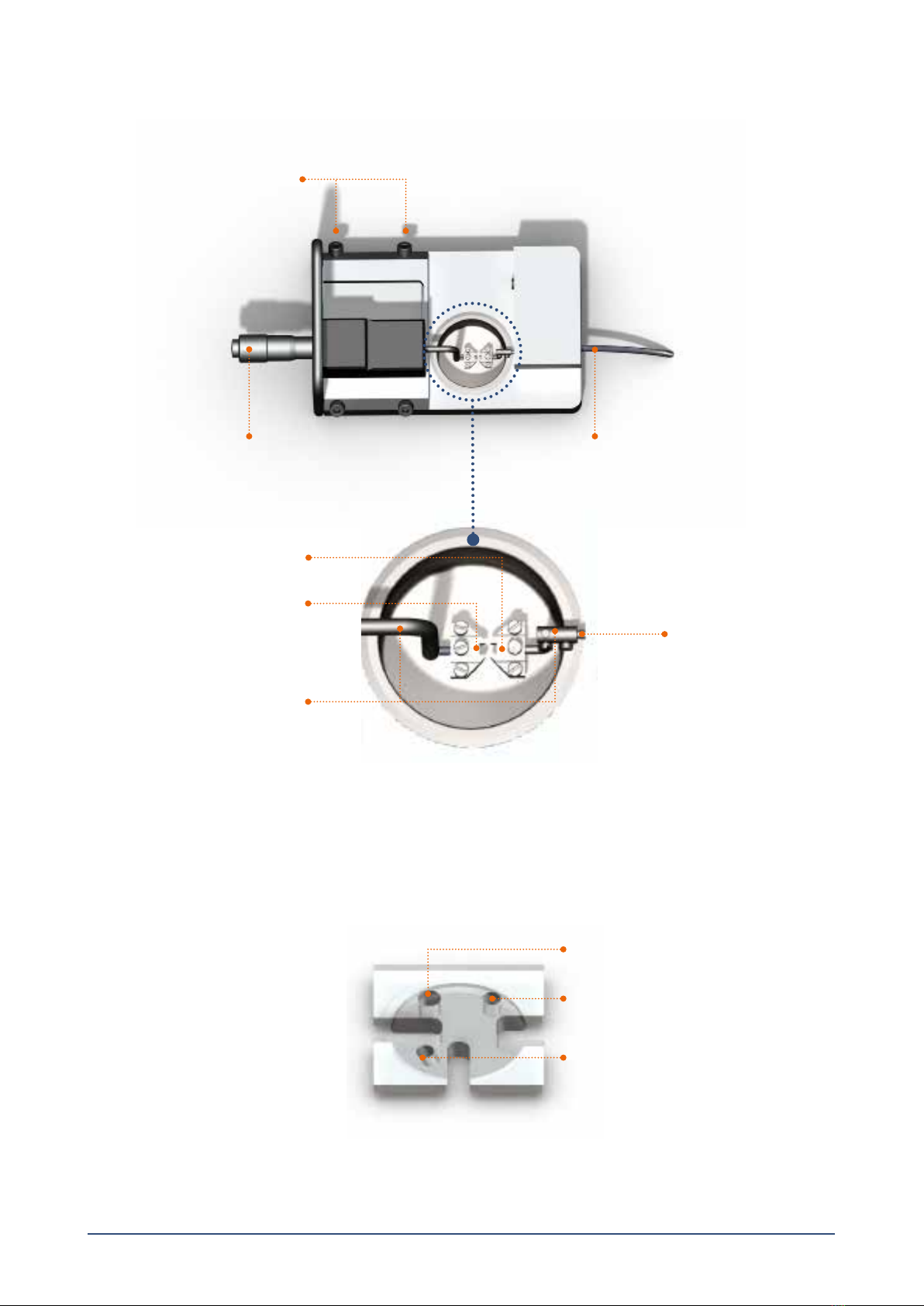
2.1 Changing and adjusting the mounting supports ...................................................................................................................................................... 4
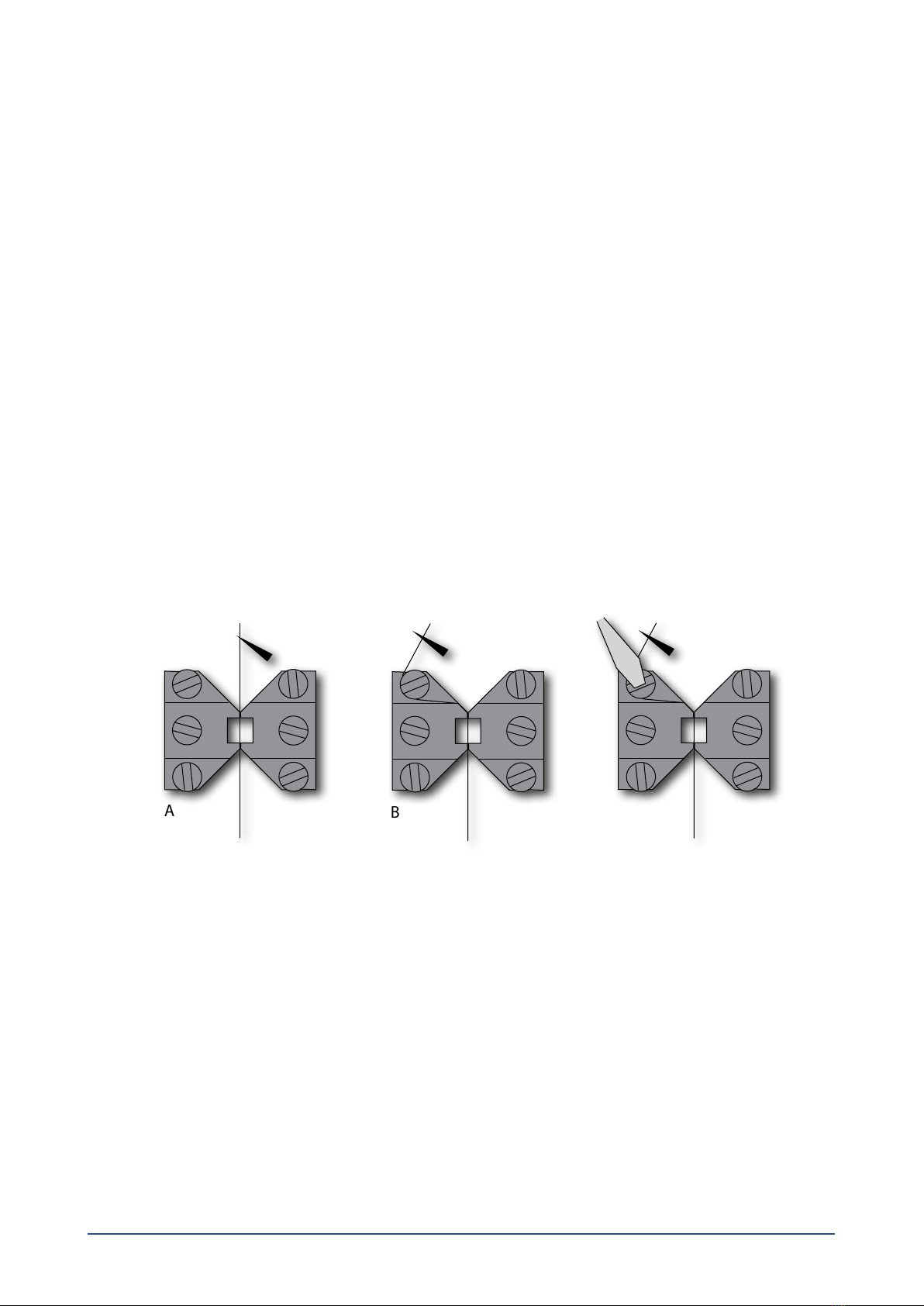
2.1.1 Changing the mounting supports (gure 2.1)......................................................................................................................................................... 4
2.1.2 Coarse adjusting the jaws for small vessels (gure 2.1)........................................................................................................................................ 4
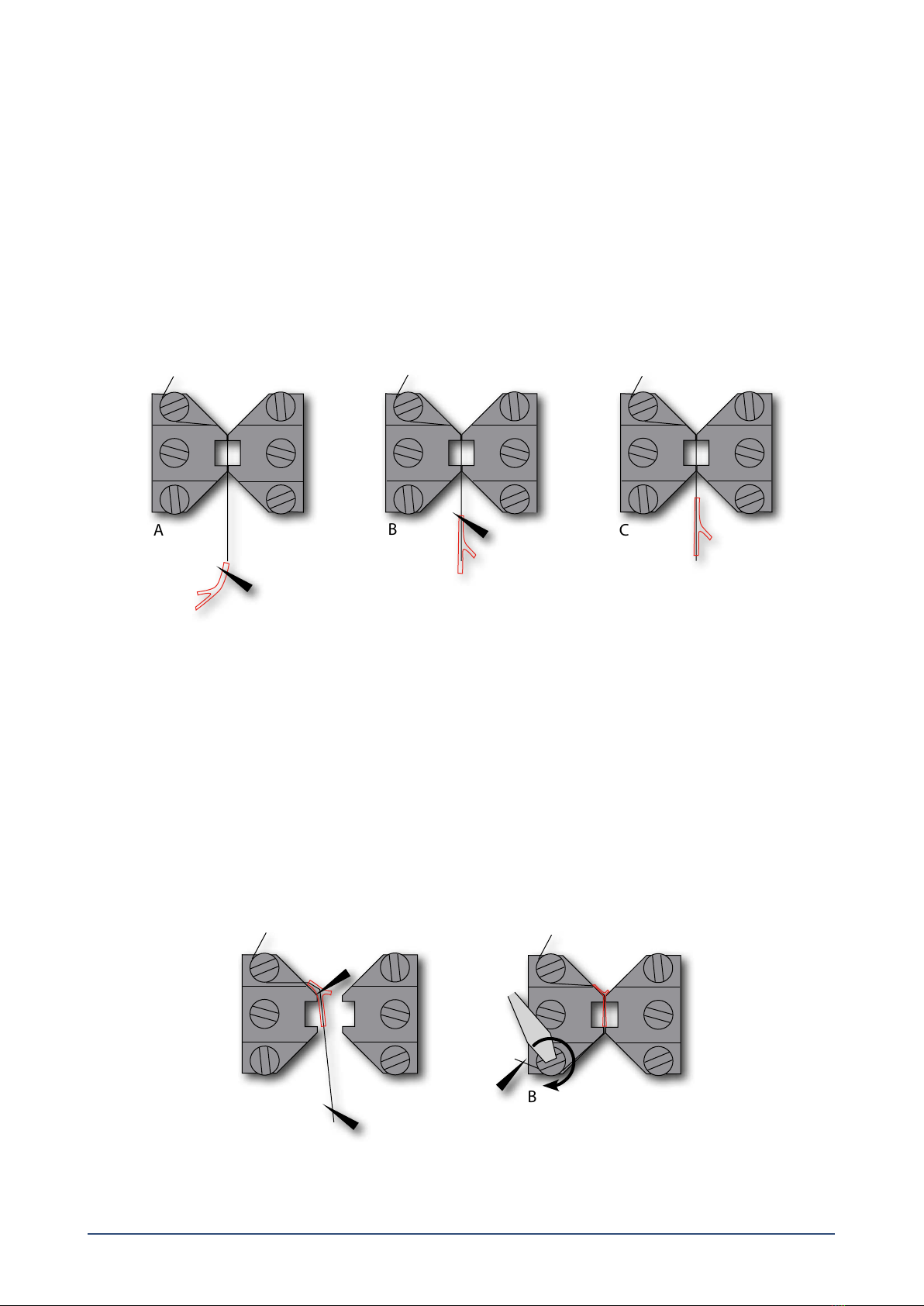
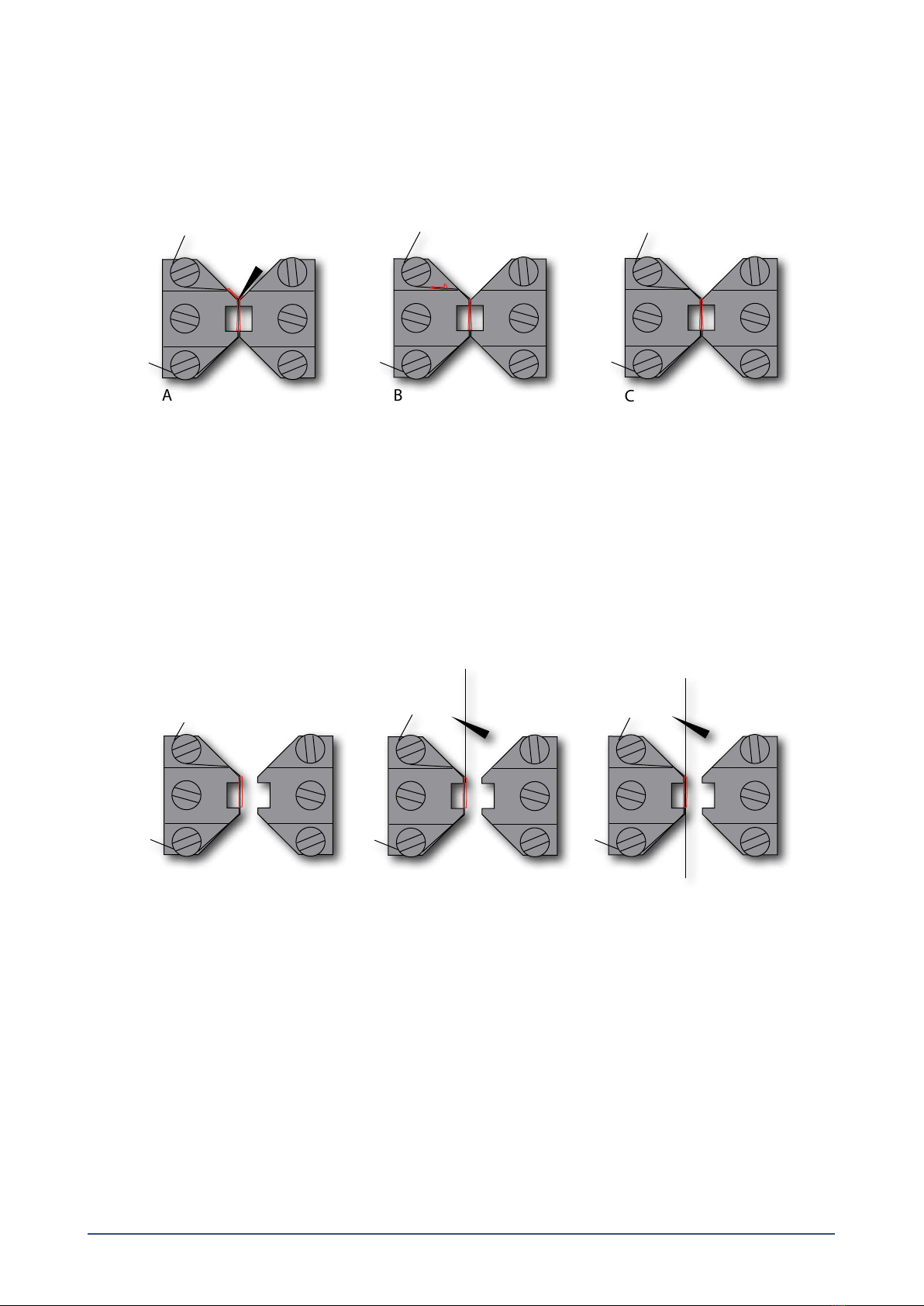
2.1.3 Fine adjusting the jaws for small vessels (gure 2.2 and gure 2.3)................................................................................................................. 5
2.1.4 Fine adjusting the pins for larger vessels (gure 2.4 and gure 2.5):............................................................................................................... 6
2.2 Calibration of the force transducer.................................................................................................................................................................................. 6
Chapter 3 - Experimental set-up ............................................................................................................................................................................................. 7
3.1 Mounting protocol for small arteries .............................................................................................................................................................................. 7
3.1.1 Mounting step one............................................................................................................................................................................................................. 7
3.1.2 Mounting step two............................................................................................................................................................................................................. 8
3.1.3 Mounting step three.......................................................................................................................................................................................................... 8
3.1.4 Mounting step four............................................................................................................................................................................................................ 9
3.1.5 Mounting step ve............................................................................................................................................................................................................. 9
3.1.6 Mounting step six.............................................................................................................................................................................................................10
3.1.7 Mounting step seven.......................................................................................................................................................................................................10
3.2 Normalization........................................................................................................................................................................................................................10
3.2.1 Principles of the normalization procedure..............................................................................................................................................................11
3.3 Standard start ........................................................................................................................................................................................................................11
3.3.1 Principles of the standard start procedure..............................................................................................................................................................11
3.4 Endothelium function ........................................................................................................................................................................................................12
3.4.1 Principles of checking endothelium function........................................................................................................................................................12
3.5 In vitro experiment 1: Noradrenaline contractile response..................................................................................................................................13
3.5.1 Background.........................................................................................................................................................................................................................13
3.5.2 Protocol ................................................................................................................................................................................................................................13
3.6 In vitro experiment 2: Acetylcholine relaxation curve............................................................................................................................................14
3.6.1 Background.........................................................................................................................................................................................................................14
3.6.2 Protocol ................................................................................................................................................................................................................................14
Chapter 4 - Cleaning and maintenance ..............................................................................................................................................................................15
4.1 Cleaning the Wire Myograph ..........................................................................................................................................................................................15
4.2 Maintenance of the force transducer............................................................................................................................................................................16
4.2.1 Checking the force transducer.....................................................................................................................................................................................16
4.2.2 Force Transducer Replacement....................................................................................................................................................................................17
4.3 Maintenance of the linear slides.....................................................................................................................................................................................18
Appendix 1 - Buer recipes .....................................................................................................................................................................................................19
Appendix 2 - Normalization theory ......................................................................................................................................................................................21
Appendix 3 - Reading a millimetre micrometer...............................................................................................................................................................23