Encision AEM EndShield User manual
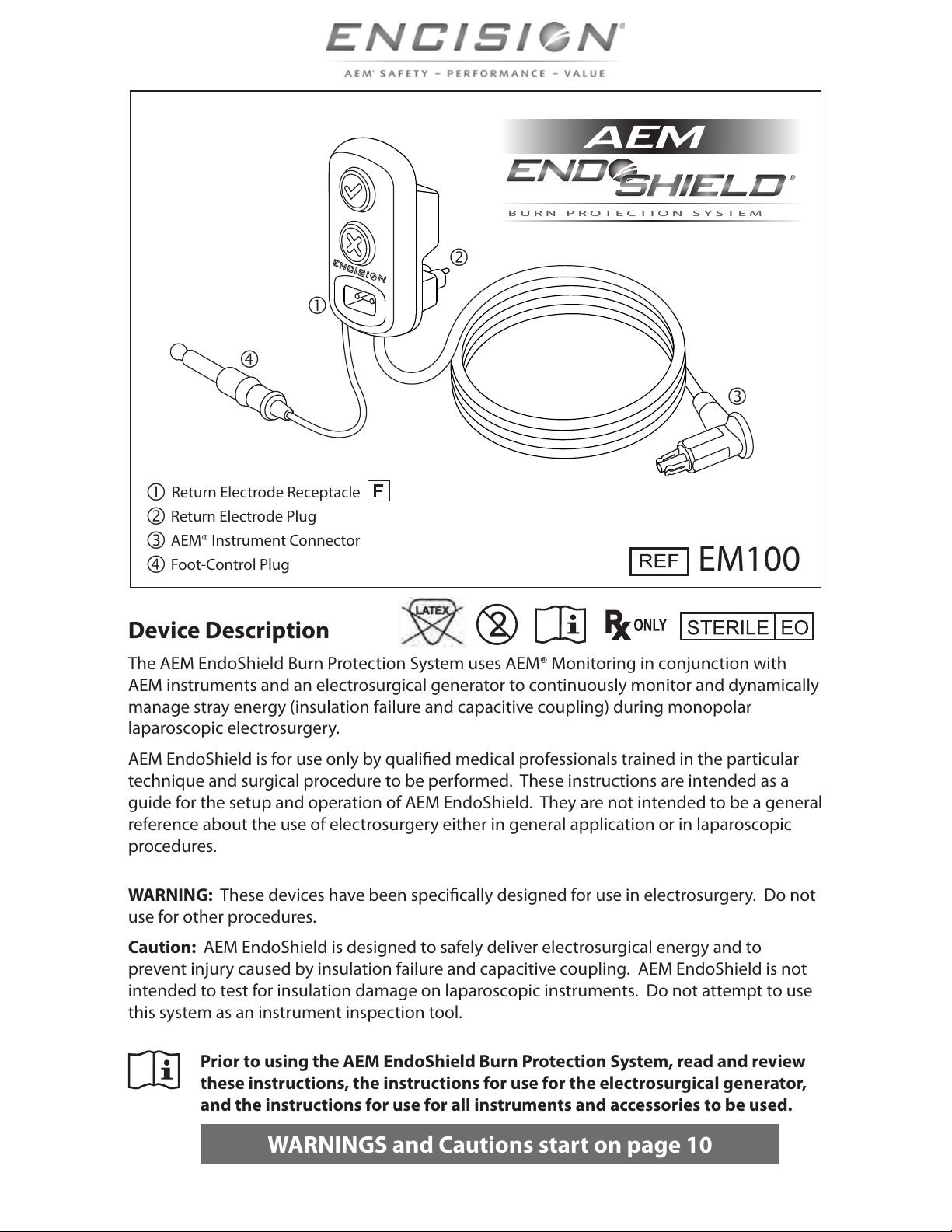
BURN PROTECTION SYSTEMBURN PROTECTION SYSTEM
EM100
Return Electrode Receptacle
Return Electrode Plug
AEM® Instrument Connector
Foot-Control Plug
Device Description
The AEM EndoShield Burn Protection System uses AEM® Monitoring in conjunction with
AEM instruments and an electrosurgical generator to continuously monitor and dynamically
manage stray energy (insulation failure and capacitive coupling) during monopolar
laparoscopic electrosurgery.
AEM EndoShield is for use only by qualied medical professionals trained in the particular
technique and surgical procedure to be performed. These instructions are intended as a
guide for the setup and operation of AEM EndoShield. They are not intended to be a general
reference about the use of electrosurgery either in general application or in laparoscopic
procedures.
WARNING: These devices have been specically designed for use in electrosurgery. Do not
use for other procedures.
Caution: AEM EndoShield is designed to safely deliver electrosurgical energy and to
prevent injury caused by insulation failure and capacitive coupling. AEM EndoShield is not
intended to test for insulation damage on laparoscopic instruments. Do not attempt to use
this system as an instrument inspection tool.
Prior to using the AEM EndoShield Burn Protection System, read and review
these instructions, the instructions for use for the electrosurgical generator,
and the instructions foruse for all instruments and accessories to be used.
WARNINGS and Cautions start on page 10

Table of Contents
How AEM Monitoring Works. . . . . . . . . . . . . . . . . . . . 3
System Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
After Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Removing the Battery . . . . . . . . . . . . . . . . . . . . 6
End of Life Indicators . . . . . . . . . . . . . . . . . . . . . 7
Reprocessing . . . . . . . . . . . . . . . . . . . . . . . . . 7
Compatible Products . . . . . . . . . . . . . . . . . . . . . . . 7
Electrosurgical Generator . . . . . . . . . . . . . . . . . . 7
Return Electrode . . . . . . . . . . . . . . . . . . . . . . . 7
Active Electrode . . . . . . . . . . . . . . . . . . . . . . . 7
Encision Adapter . . . . . . . . . . . . . . . . . . . . . . . 7
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . 8
Mechanical Inspection . . . . . . . . . . . . . . . . . . . . 8
Correcting Setup Faults. . . . . . . . . . . . . . . . . . . . 8
Responding to AEM EndoShield Alarms. . . . . . . . . . . . 9
Warnings and Cautions . . . . . . . . . . . . . . . . . . . . . . 10
Technical Specications . . . . . . . . . . . . . . . . . . . . . . 12
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . 17
Symbol Denitions . . . . . . . . . . . . . . . . . . . . . . . . 19
Indications for Use
The AEM EndoShield Burn Protection System is an accessory for use with
electrosurgical generators and AEM instruments that is designed to safely deliver
electrosurgical energy and to prevent injury caused by insulation failure and
capacitive coupling.
Active electrode monitoring is intended to control stray monopolar energy caused
by insulation failure and capacitive coupling in surgical instruments on the shaft of
the instrument.
Contraindications
There are no known contraindications for the use of AEM EndoShield.
AEM EndoShield 3 of 39
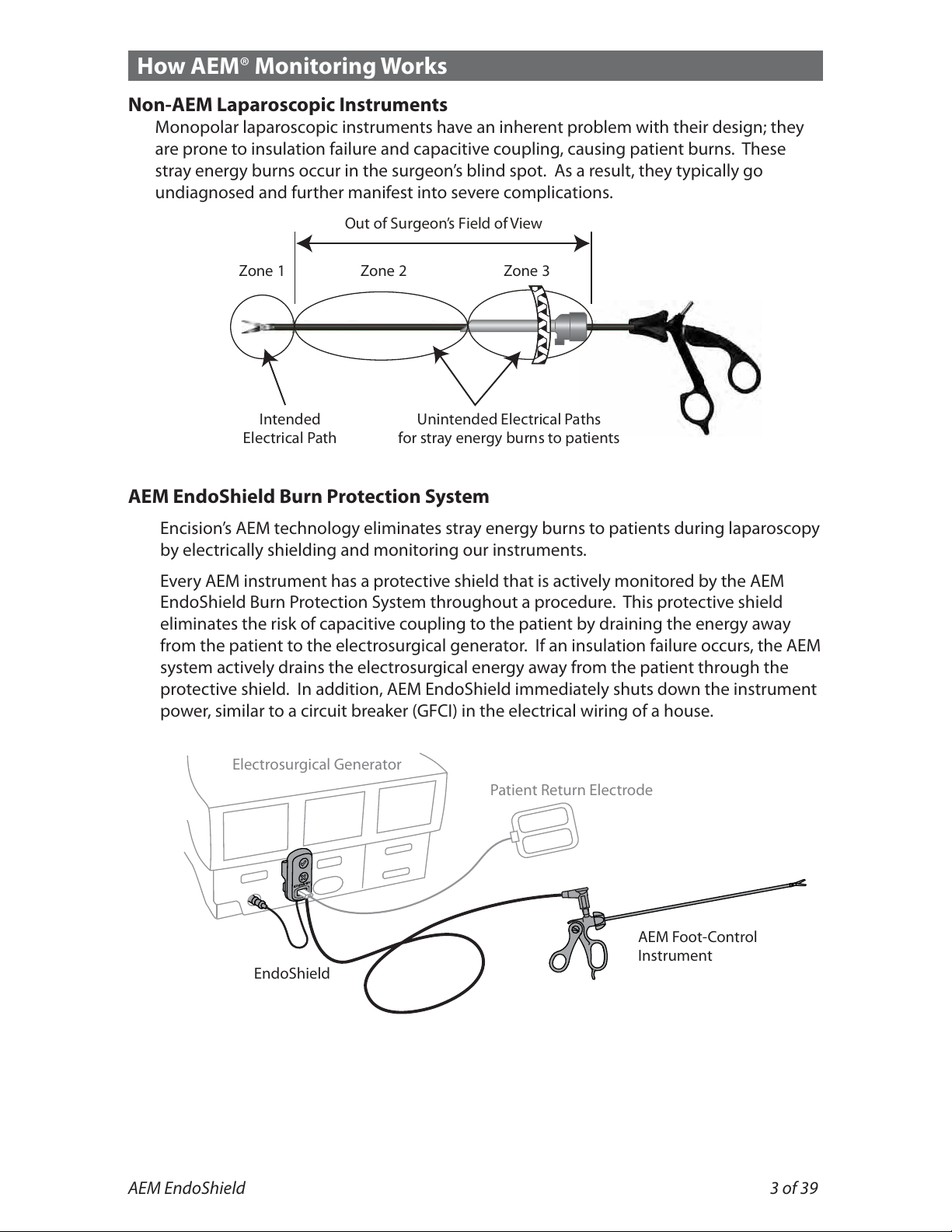
How AEM®Monitoring Works
Non-AEM Laparoscopic Instruments
Monopolar laparoscopic instruments have an inherent problem with their design; they
are prone to insulation failure and capacitive coupling, causing patient burns. These
stray energy burns occur in the surgeon’s blind spot. As a result, they typically go
undiagnosed and further manifest into severe complications.
AEM EndoShield Burn Protection System
Encision’s AEM technology eliminates stray energy burns to patients during laparoscopy
by electrically shielding and monitoring our instruments.
Every AEM instrument has a protective shield that is actively monitored by the AEM
EndoShield Burn Protection System throughout a procedure. This protective shield
eliminates the risk of capacitive coupling to the patient by draining the energy away
from the patient to the electrosurgical generator. If an insulation failure occurs, the AEM
system actively drains the electrosurgical energy away from the patient through the
protective shield. In addition, AEM EndoShield immediately shuts down the instrument
power, similar to a circuit breaker (GFCI) in the electrical wiring of ahouse.
Zone 1 Zone 2 Zone 3
Out of Surgeon’s Field of View
Unintended Electrical Paths
for stray energy burns to patients
Intended
Electrical Path
Patient Return Electrode
Electrosurgical Generator
EndoShield
AEM Foot-Control
Instrument
4 of 39
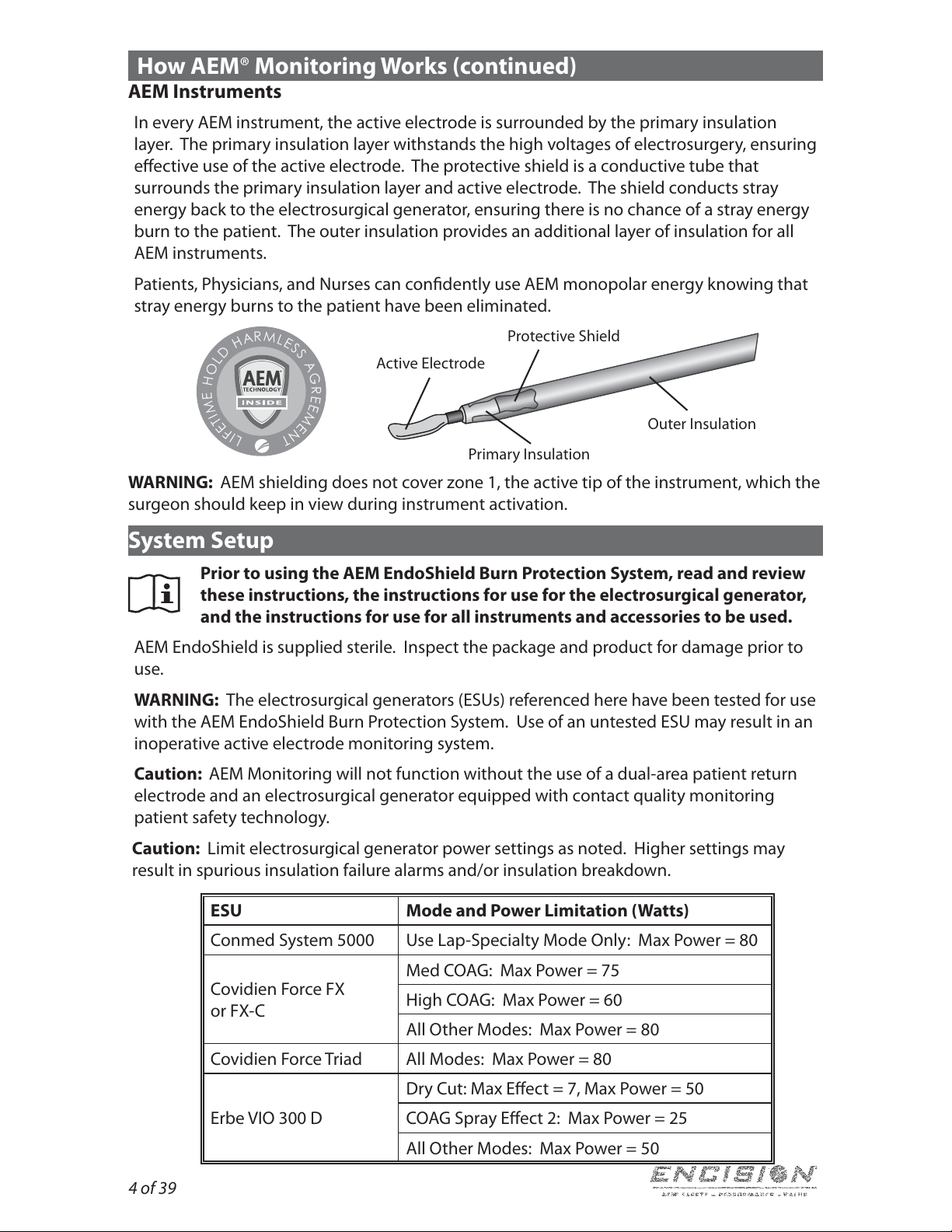
How AEM®Monitoring Works (continued)
AEM Instruments
In every AEM instrument, the active electrode is surrounded by the primary insulation
layer. The primary insulation layer withstands the high voltages of electrosurgery, ensuring
eective use of the active electrode. The protective shield is a conductive tube that
surrounds the primary insulation layer and active electrode. The shield conducts stray
energy back to the electrosurgical generator, ensuring there is no chance of a stray energy
burn to the patient. The outer insulation provides an additional layer of insulation for all
AEMinstruments.
Patients, Physicians, and Nurses can condently use AEM monopolar energy knowing that
stray energy burns to the patient have been eliminated.
Protective Shield
Active Electrode
Primary Insulation
Outer Insulation
WARNING: AEM shielding does not cover zone 1, the active tip of the instrument, which the
surgeon should keep in view during instrument activation.
System Setup
Prior to using the AEM EndoShield Burn Protection System, read and review
these instructions, the instructions for use for the electrosurgical generator,
and the instructions for use for all instruments and accessories to be used.
AEM EndoShield is supplied sterile. Inspect the package and product for damage prior to
use.
WARNING: The electrosurgical generators (ESUs) referenced here have been tested for use
with the AEM EndoShield Burn Protection System. Use of an untested ESU may result in an
inoperative active electrode monitoring system.
Caution: AEM Monitoring will not function without the use of a dual-area patient return
electrode and an electrosurgical generator equipped with contact quality monitoring
patient safety technology.
Caution: Limit electrosurgical generator power settings as noted. Higher settings may
result in spurious insulation failure alarms and/or insulation breakdown.
ESU Mode and Power Limitation (Watts)
Conmed System 5000 Use Lap-Specialty Mode Only: Max Power = 80
Covidien Force FX
or FX-C
Med COAG: Max Power = 75
High COAG: Max Power = 60
All Other Modes: Max Power = 80
Covidien Force Triad All Modes: Max Power = 80
Erbe VIO 300 D
Dry Cut: Max Eect = 7, Max Power = 50
COAG Spray Eect 2: Max Power = 25
All Other Modes: Max Power = 50
AEM EndoShield 5 of 39
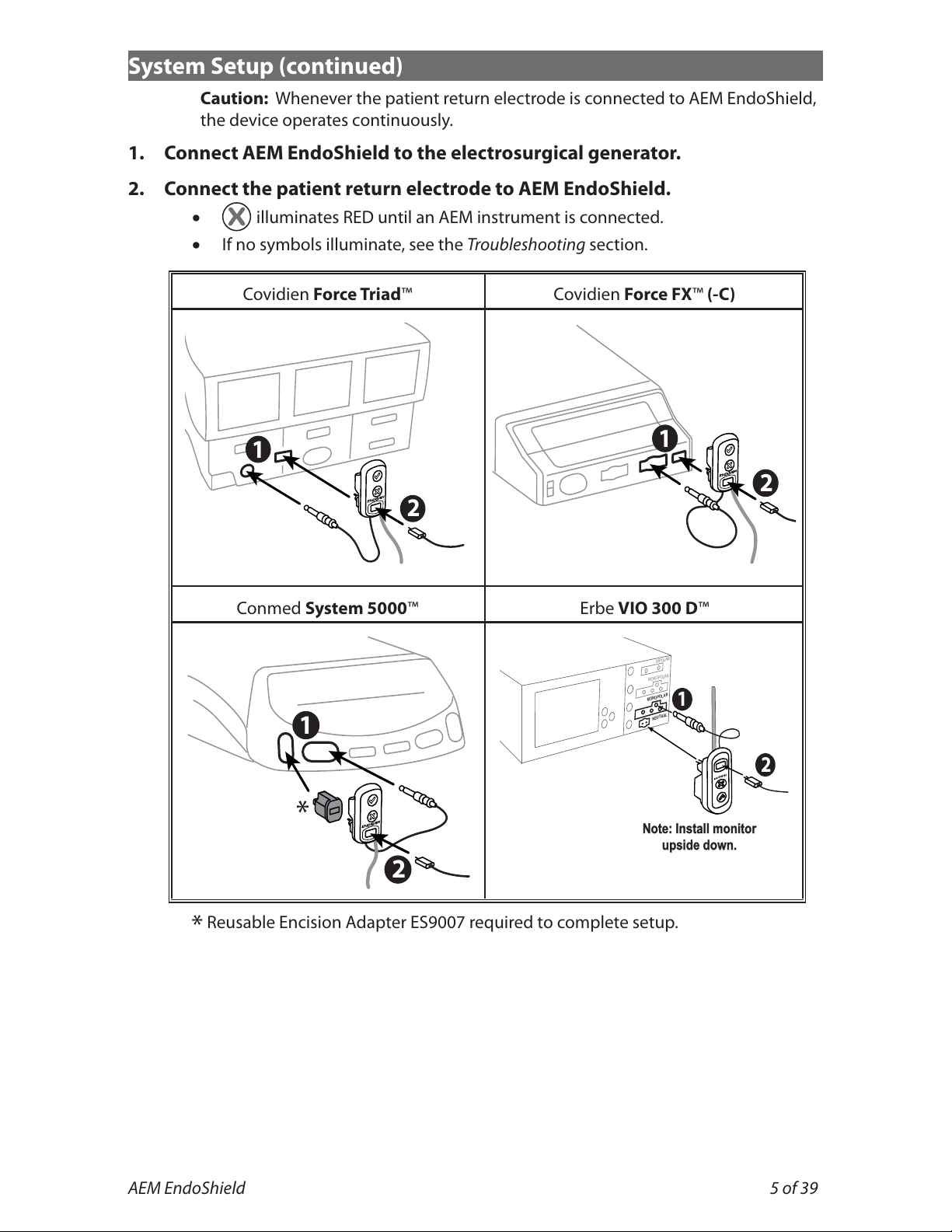
System Setup (continued)
Caution: Whenever the patient return electrode is connected to AEM EndoShield,
the device operates continuously.
1. Connect AEM EndoShield to the electrosurgical generator.
2. Connect the patient return electrode to AEM EndoShield.
illuminates RED until an AEM instrument is connected.
If no symbols illuminate, see the Troubleshooting section.
Covidien Force Triad™ Covidien Force FX™(-C)
1
2
1
2
Conmed System 5000™ Erbe VIO 300 D™
2
1
*
1
BIPOLAR
MONOPOLAR
MONOPOLAR
N
EUTRAL
2
Note: Install monitor
Note: Install monitor
upside down.
upside down.
*Reusable Encision Adapter ES9007 required to complete setup.
6 of 39
System Setup (continued)
3. Connect an AEM foot-control instrument to AEM EndoShield.
illuminates GREEN when the AEM EndoShield Burn Protection System is
fully operational.
If illuminates RED, see the Troubleshooting section.
3
4. Turn on the electrosurgical generator, enabling its contact quality
monitoring system. It should be in its normal operating state.
After Use
Caution: This product is supplied sterile and is not intended for use more than
one time. No attempt should be made to reprocess this device.
Caution: Used instruments are considered medical waste. Dispose of in
accordance with local regulations.
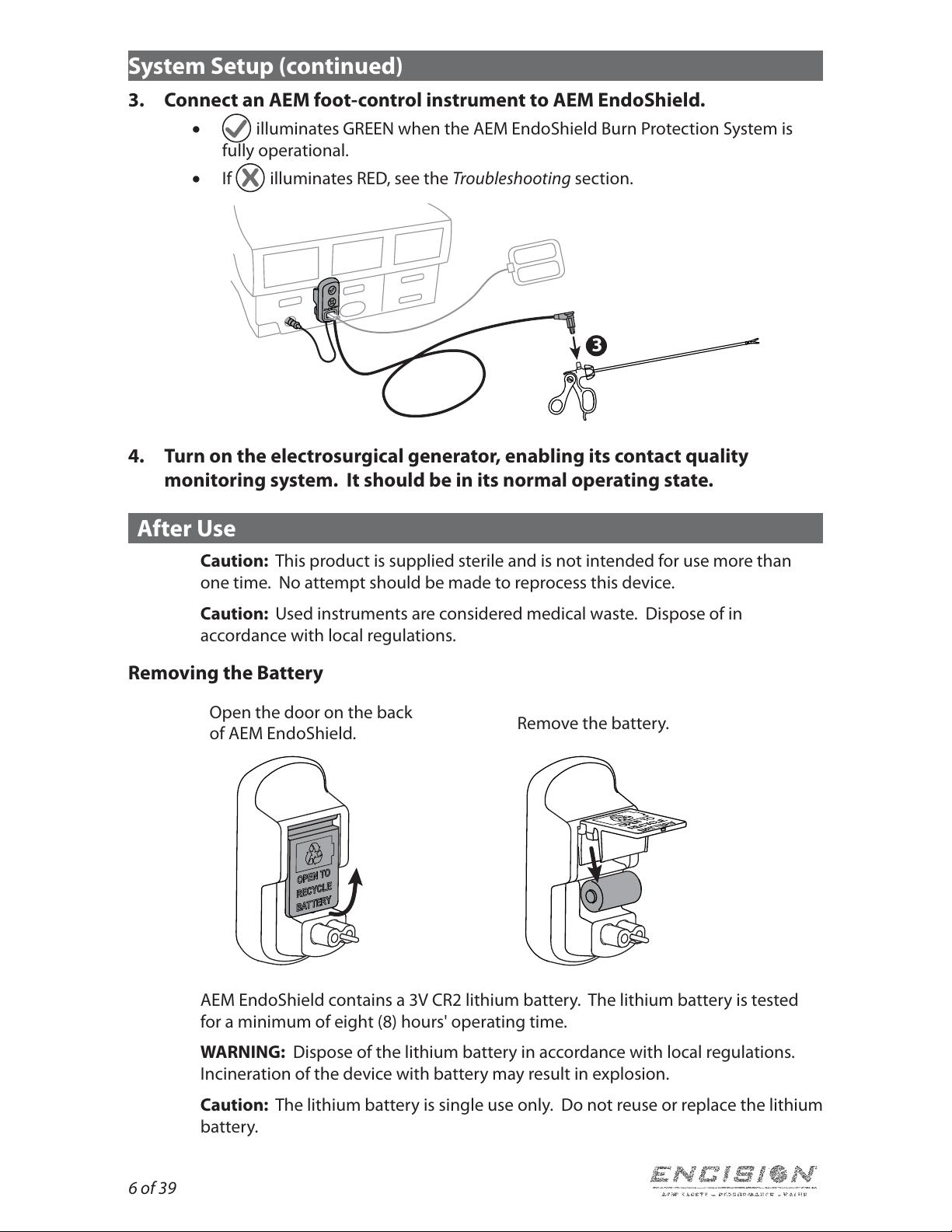
Removing the Battery
Open the door on the back
of AEM EndoShield. Remove the battery.
AEM EndoShield contains a 3V CR2 lithium battery. The lithium battery is tested
for a minimum of eight (8) hours' operating time.
WARNING: Dispose of the lithium battery in accordance with local regulations.
Incineration of the device with battery may result in explosion.
Caution: The lithium battery is single use only. Do not reuse or replace the lithium
battery.
AEM EndoShield 7 of 39
After Use (continued)
End of Life Indicators
Discontinue use if any of the following are evident:
intermittent electrical performance
after one use of product
Reprocessing
WARNING: This product is intended for single use and shall not be reprocessed or
resterilized. Resterilization may result in explosion or re hazard. Resterilization may
compromise the integrity of the device, which may result in malfunction or electrical
hazard to the patient or user.
Compatible Products
For successful operation, the AEM EndoShield Burn Protection System must be used with
the following compatible products.
Caution: Use of other accessories or cables may result in increased EMC emissions or
decreased immunity.
Electrosurgical Generator
Electrosurgical Generator (ESU) Encision Adapter
Required
Manufacturer Model
Conmed System 5000 ES9007
Covidien
Force Triad N/A
Force FX N/A
Force FX-C N/A
Erbe VIO 300 D™ N/A
WARNING: The electrosurgical generators referenced here have been tested for use with
the AEM EndoShield Burn Protection System. Use of an untested ESU may result in an
inoperative active electrode monitoring system.
Caution: All electrosurgical generators must have a contact quality monitoring circuit for
return electrodes.
Return Electrode
The AEM EndoShield Burn Protection System requires a dual-area patient return electrode.
Active Electrode
The foot-control instrument must have patented AEM technology and be manufactured
by/for Encision Inc., or licensed by Encision Inc.
Hand-control AEM instruments are not compatible with the AEM EndoShield Burn
Protection System.
Encision Adapter
Some electrosurgical generators require an adapter for setup of the AEM EndoShield Burn
Protection System (see the Electrosurgical Generator table above). Refer to the Setup
section for proper connections.
8 of 39
Troubleshooting
Mechanical Inspection
Before use, visually inspect the following items of AEM EndoShield. Donot use
if any of these items appear damaged:
insulation of wiring and cables
instrument receptacles and connectors
Correcting Setup Faults
Verify that the setup of the AEM EndoShield Burn Protection System is complete.
Situation Recommended Action
No symbols illuminate on the
front of AEM EndoShield.
Verify that a dual-area patient return electrode
is connected properly and fully seated in
the return electrode receptacle of AEM
EndoShield.
If the fault persists, replace the patient return
electrode.
Check the Use By date on the AEM EndoShield
package. If the product is beyond the
specied date, the battery life may be low or
depleted, replace AEM EndoShield.
illuminates RED on AEM
EndoShield, continuous.
Verify that the AEM instrument is properly
connected to AEM EndoShield.
If the fault persists, replace the AEM
instrument.
No power to instrument,
but the illuminates GREEN
on AEM EndoShield.
Verify that both AEM EndoShield connectors
to the electrosurgical generator (ESU) are
properly and securely connected.
Ensure that the power settings on the ESU are
sucient
Verify that the foot pedal is properly
connected to the ESU.
Reset the ESU's pad monitoring system
(applies to some ESU models).
Check the return electrode application to
the patient. Follow the return electrode
manufacturer’s instructions for proper
placement.
If the fault persists after performing all the
previous steps, replace the AEM instrument.
If the fault continues to persist, replace AEM
EndoShield.
AEM EndoShield 9 of 39
Troubleshooting (continued)
Responding to AEM EndoShield Alarms
When using AEM Monitoring, successful electrosurgery depends on an absence of
critical fault conditions. Should one occur, the AEM EndoShield Burn Protection
System interrupts the power delivery from the electrosurgical generator to the
AEM instrument for 10seconds.
If for any reason a fault condition persists from AEM EndoShield after following
the steps described below, use a backup AEM EndoShield to complete the surgical
procedure.
Situation Recommended Action
illuminates RED on AEM
EndoShield for 10seconds.
AEM EndoShield interrupts the
circuit to the electrosurgical
generator for 10 seconds.
Verify that the AEM instrument is properly
connected to AEM EndoShield.
Activate the instrument.
If the continues to illuminate RED, replace
the instrument.
If the continues to illuminate RED after
replacing the instrument, replace AEM
EndoShield.
illuminates RED on the
AEM EndoShield, continuous.
AEM EndoShield interrupts the
circuit to the electrosurgical
generator until the alarm
condition is satised.
Disconnect the AEM instrument from
EndoShield.
Reconnect the AEM instrument to AEM
EndoShield.
If the continues to illuminate RED, replace
the instrument.
If the continues to illuminate RED after
replacing the instrument, replace AEM
EndoShield.
WARNING: An AEM EndoShield alarm may indicate an unsafe condition.
Electrosurgical energy to the AEM instrument is disabled during the alarm
condition.
If other alert conditions occur during the surgical procedure, see Correcting Setup
Faults on previous page.

10 of 39
Warnings and Cautions
Prior to using the AEM EndoShield Burn Protection System, read and review
these instructions, the instructions for use for the electrosurgical generator,
and the instructions for use for all instruments and accessories to be used.
Fire and Shock Hazards
WARNING:
Explosion Hazard. Do not use electrosurgery in the presence of ammable
liquids or in an oxygen enriched environment.
Fire Hazard. Electrosurgical accessories that are activated or hot from use can
cause a re. Do not place them near or touching ammable materials (such as
gauze or surgical drapes).
Electric Shock Hazard. Ensure that all accessories, cords, and adapters are
correctly connected.
Electric Shock Hazard. Do not attempt to connect or disconnect any cable
during power activation.
General
WARNING:
Conrm proper electrosurgical power setting before proceeding with surgery.
Use the lowest power setting that achieves the desired surgical eect.
Keep electrical connections dry while in use to prevent potential conduction of
High Frequency (HF) current to the user.
Only an AEM instrument provides active electrode monitoring. Other
conductive objects at or near the surgical site are not protected. Do not touch
those objects with the active instrument.
No modication of this equipment is allowed.
Caution:
Limit electrosurgical generator power settings as noted in the ESU Mode and
Power Limitation table in the System Setup section. Higher settings may result
in spurious insulation failure alarms and/or insulation breakdown.
Active Accessories and the AEM EndoShield Burn Protection System
WARNING:
Do not wrap accessory cords around metal objects. Wrapping cords around
metal objects may induce currents that could lead to shocks, res, or injury.
The electrode tip may remain hot enough to cause burns after the
electrosurgical current is deactivated.
When not in use, place accessories in a clean, dry, nonconductive and highly
visible area not touching the patient. Inadvertent contact with the patient
may result in burns.
Ensure that the insulation of conventional, nonshielded disposable and
reusable laparoscopic instrumentation is intact. Compromised insulation of
nonshielded instruments may lead to shocks or burns to the patient or surgical
personnel.

AEM EndoShield 11 of 39
Warnings and Cautions (continued)
When using laparoscopic instrumentation with metal cannulas, the potential
exists for abdominal wall burns to occur in the event of direct electrode tip
contact to the cannula.
Inspect cords for breaks, cracks, nicks. If any are present, do not use. Failure to
observe this precaution may result in injury or electrical shock to the patient or
operating personnel.
Damaged external insulation on instruments AND incorrect setup of the AEM
EndoShield Burn Protection System may result in a risk of unintended patient
burn. Do not use product having damaged insulation.
When an alert is presented by AEM EndoShield, discontinue use of the
electrosurgical current immediately. Find the cause of the alert and correct it
before continuing use.
Damaged internal insulation of the instrument, or loss of shield continuity, may
cause AEM EndoShield alarms. For maximum patient safety, discontinue use of
the instrument if this occurs.
A single AEM instrument must be the sole conductor of energy to tissue. Do
not conduct energy by touching an AEM instrument to a second instrument
contacting tissue. The second device will not be protected from capacitive
coupling and insulation failure.
Caution:
Read the instructions, warnings, and cautions provided with the AEM
EndoShield Burn Protection System accessories before using. Their specic
instructions are not included in this manual.
AEM Monitoring will not function without the use of a dual-area patient return
electrode and an electrosurgical generator equipped with contact quality
monitoring patient safety technology.
This product is supplied sterile and is not intended for use more than one time.
No attempt should be made to reprocess this device.
Electromagnetic Compatibility (EMC) Hazards
For EMC specication tables, refer to the Technical Specications section.
Caution:
Use of accessories, transducers, and cables other than those specied, with
the exception of transducers and cables sold by the manufacturer of the
Equipment or System as replacement parts for internal components, may
result in increased Emissions or decreased Immunity of the Equipment
orSystem.

12 of 39
Technical Specications
Monopolar Operating Mode
The AEM EndoShield Burn Protection System detects improper setup conditions
and detects operative faults by providing a monitored pathway for the current
which is owing from the shield to the patient return electrode. The fault
condition is indicated on the front of AEM EndoShield and the ow of energy is
interrupted from the electrosurgical generator to the AEM instrument.
Functional Characteristics
Monopolar Setup Fault Detection
If the return electrode is disconnected or the wrong type of return electrode is
connected, no symbols illuminate on the front of AEM EndoShield.
If an AEM foot-control instrument is not connected to AEM EndoShield or not
connected properly, the illuminates RED to identify the setup fault.
Shield Cable and Return Electrode Switch Threshold
50 ohms ± 40%
Monopolar Operative Fault Detection
If there is excessive shield current or arcing between the shield and the active
electrode causing an operative fault, the illuminates RED to identify an
insulation fault, and the AEM EndoShield Burn Protection System interrupts the
ow of energy from the electrosurgical generator to the AEM instrument.
Radio Frequency Current Sensing
Current-sensing and spark detection are provided. Minimum electrosurgical
generator output for reliable insulation fault detection: 20 Watts.
Indicators and Alert Functions
GREEN Indicates that the AEM EndoShield Burn Protection System is
fully operational.
RED During setup, indicates that the AEM instrument is not properly
connected.
During use, indicates that the instrument in use has an unsafe
operating condition. Indicates that there is excessive current
or arcing between the active electrode and the shield. Once
triggered in this condition, illuminates RED for 10 seconds.

AEM EndoShield 13 of 39
Technical Specications (continued)
Connectors and Cables
Return Electrode Receptacle
A dual pin receptacle on the front of AEM EndoShield. Connects a dual-area
patient return electrode to AEM EndoShield.
Return Electrode Plug
A single pin plug on the rear of AEM EndoShield. Connects to the electrosurgical
generator's patient return electrode receptacle.
AEM Instrument Connector
Connects the AEM instrument active conductor (foot-control instrument only) to
the electrosurgical generator and the shield conductors to AEM EndoShield.
Foot-Control Plug
A single active pin that connects AEM EndoShield to the electrosurgical
generator’s footswitch accessory receptacle.
Maximum Electrosurgical Generator Voltage
4.1 KVpeak
Electrical Characteristics
Power Source
Lithium Battery, 3V CR2
Patient Leakage Current
Source or sink leakage current is 10 µA maximum
Dimensions and Weight
External Dimensions
4” tall x 2” wide x 2.5” deep, excluding integrated cords
AEM Instrument Connector: 9' cord length
Foot-Control Plug: 6" cord length
Weight
8 oz. (227 g), excluding ESU-required adapters
Environmental Characteristics
Operating Temperature
59 to 104° F (15 to 40° C)
Storage and Transport Temperature
-13 to 140° F (-25 to 60° C)
Operating, Storage and Transport Humidity
5% to 95% relative, non-condensing
Atmospheric Pressure (Operating)
70 - 110 kPa
14 of 39
Technical Specications (continued)
Standards and IEC Classications
Internally Powered Equipment per IEC 60601-1/EN 60601-1
Equipment operates from an internal electrical power source.
Debrillator Protected Equipment
The electrosurgical generator provides debrillator protection. AEM Endoshield
does not compromise this protection.
Caution: Ordinary equipment is not protected against the ingress of water.
Caution: Medical Electrical Equipment needs special precautions regarding
electromagnetic compatibility (EMC) and needs to be installed and put into service
according to the EMC information provided herein.
Caution: Portable and mobile RF communications equipment can aect Medical
Electrical Equipment.
Electromagnetic emissions and immunity per IEC 60601-1-2/EN 60601-1-2
Guidance and Manufacturer’s Declaration –Emissions
The Encision AEM EndoShield Burn Protection System is intended for use in the electromagnetic
environment specied below. The customer or user of AEM EndoShield should ensure that it is used
in such an environment.
Emissions Test Compliance Electromagnetic Environment –Guidance
RF emissions
CISPR 11
Group 1 AEM EndoShield uses RF energy only for its
internal function. Therefore, its RF emissions
are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emissions
CISPR 11
Class B AEM EndoShield is suitable for use in all
establishments, including domestic, and those
directly connected to the public low-voltage
power supply network that supplies buildings
used for domestic purposes.
Harmonics
IEC 61000-3-2
N/A
Flicker
IEC 61000-3-3
N/A

AEM EndoShield 15 of 39
Guidance and Manufacturer’s Declaration –Immunity
The Encision AEM EndoShield Burn Protection System is intended for use in the electromagnetic
environment specied below. The customer or user of AEM EndoShield should ensure that it is used
in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment –
Guidance
Electrostatic
Discharge (ESD)
IEC 61000-4-2
±6kV Contact
±8kV Air
±6kV Contact
±8kV Air
Floors should be wood, concrete or
ceramic tile. If oors are synthetic,
the r/h should be at least 30%.
Electrical Fast
Transient (EFT)
IEC 61000-4-4
±2kV Mains
±1kV Input/Output
(I/Os)
N/A Mains power quality should be
that of a typical commercial or
hospital environment.
Surge
IEC 61000-4-5
±1kV Dierential
±2kV Common
N/A Mains power quality should be
that of a typical commercial or
hospital environment.
Voltage Dips/
Dropout
IEC 61000-4-11
>95% Dip for
0.5 Cycle
60% Dip for
5 Cycles
30% Dip for
25 Cycles
>95% Dip for
5 Seconds
N/A Mains power quality should be that
of a typical commercial or hospital
environment. If the user of AEM
EndoShield requires continued
operation during power mains
interruptions, it is recommended
that AEM EndoShield be powered
from an uninterruptible power
supply or a battery.
Power
Frequency
50/60Hz
Magnetic Field
IEC 61000-4-8
3A/m 3A/m Power frequency magnetic
elds should be that of a
typical commercial or hospital
environment.
Technical Specications (continued)
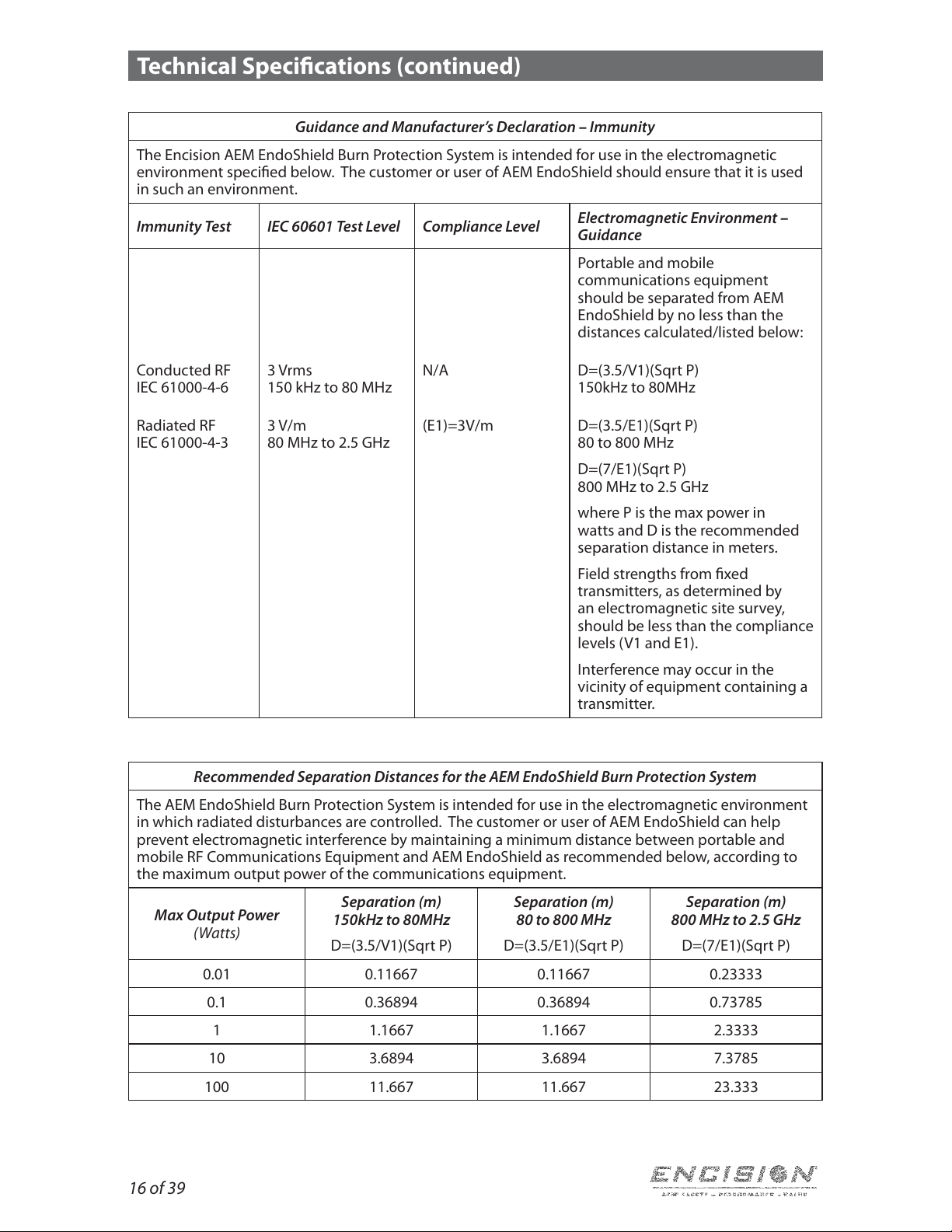
16 of 39
Guidance and Manufacturer’s Declaration –Immunity
The Encision AEM EndoShield Burn Protection System is intended for use in the electromagnetic
environment specied below. The customer or user of AEM EndoShield should ensure that it is used
in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment –
Guidance
Portable and mobile
communications equipment
should be separated from AEM
EndoShield by no less than the
distances calculated/listed below:
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
N/A D=(3.5/V1)(Sqrt P)
150kHz to 80MHz
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2.5 GHz
(E1)=3V/m D=(3.5/E1)(Sqrt P)
80 to 800 MHz
D=(7/E1)(Sqrt P)
800 MHz to 2.5 GHz
where P is the max power in
watts and D is the recommended
separation distance in meters.
Field strengths from xed
transmitters, as determined by
an electromagnetic site survey,
should be less than the compliance
levels (V1 and E1).
Interference may occur in the
vicinity of equipment containing a
transmitter.
Recommended Separation Distances for the AEM EndoShield Burn Protection System
The AEM EndoShield Burn Protection System is intended for use in the electromagnetic environment
in which radiated disturbances are controlled. The customer or user of AEM EndoShield can help
prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF Communications Equipment and AEM EndoShield as recommended below, according to
the maximum output power of the communications equipment.
Max Output Power
(Watts)
Separation (m)
150kHz to 80MHz
D=(3.5/V1)(Sqrt P)
Separation (m)
80 to 800 MHz
D=(3.5/E1)(Sqrt P)
Separation (m)
800 MHz to 2.5 GHz
D=(7/E1)(Sqrt P)
0.01 0.11667 0.11667 0.23333
0.1 0.36894 0.36894 0.73785
1 1.1667 1.1667 2.3333
10 3.6894 3.6894 7.3785
100 11.667 11.667 23.333
Technical Specications (continued)

AEM EndoShield 17 of 39
Limited Warranty
Express Warranty: ENCISION hereby warrants to Buyer that products purchased
hereunder shall be free from defects in material and workmanship under normal
use and service, as specied in ENCISION’s product manuals and Instructions for
Use provided with such product, for the period of:
AEM EndoShield Burn Protection System – until the labeled USE BY date, or
one (1) use, whichever occurs rst.
Instruments and Accessories – as stated in the applicable Instructions for Use.
This warranty shall run in favor of Buyer only, and is not enforceable by any
other person or entity.
Disclaimer: The express warranties set forth in this agreement are in lieu of,
and buyer hereby expressly waives, all other guarantees and warranties of
any kind, whether express, implied or statutory including, without limitation,
merchantability, tness for particular purpose, non-infringement or by sample, and
all such other warranties are hereby disclaimed and excluded by ENCISION. The
sole and exclusive remedy for breach of ENCISION’s warranty of the products shall
be as stated herein.
Exclusions: The express warranty set forth above specically excludes and does
not apply to defects (i) caused through no fault of ENCISION during shipment to
or from Buyer, (ii) caused by modications or alterations made to the products
by Buyer or any third party (iii) caused by unauthorized repair or maintenance
performed on the products by Buyer or any third party, (iv) caused by the failure of
Buyer to comply with any of the return procedures specied below, or (v) damaged
by excessive current, temperature, physical stress or other deviation from the
applicable environmental specications.
Limitation of Remedies: ENCISION’s sole obligation and Buyer’s exclusive remedy
for any breach of warranty is limited to the repair or replacement, at Encision’s
option, of any warranted product that is returned to ENCISION in its standard
shipping container or properly packed in accordance with ENCISION’s packing
procedures, freight prepaid, where ENCISION’s examination shows the product
to have failed under normal use. If ENCISION’s examination discloses that the
returned product is not defective within the terms of this warranty, Buyer shall
be subject to a $200.00 charge per individual product for testing expenses
incurred by ENCISION and the product will be returned to Buyer, freight collect.
Such repair or replacement and reshipment at ENCISION’s expense will be
Buyer’s sole and exclusive remedy for such defect. ENCISION will pay shipping
charges for the repaired or replaced from ENCISION’s factory to Buyer’s location.
If,notwithstanding the foregoing, Buyer ships any product to ENCISION’s factory
freight collect, then ENCISION shall ship the repaired or replaced product freight
collect.
Warranty Procedures: Buyer shall request authorization from ENCISION prior to
the return of each defective product for repair or replacement by ENCISION. Upon
such request, ENCISION shall provide the address of the facility to which such
product must be returned, together with Return Material Authorization (RMA)
tracer number. ENCISION may, at its sole option, employ new or used parts for
products to make such repair or replacement.

18 of 39
Limited Warranty (continued)
Stored Data: ENCISION shall not be liable for any loss or damage to any data
stored in any product, including, without limitation, any data loss or damage
resulting from any malfunction or defect or any loss or damage resulting from
any inspection, repair, refurbishment, reconditioning or testing of the product
or incurred in connection with transportation of the product to ENCISION or
ENCISION’s authorized repair center.
Technical Assistance: ENCISION’s warranty shall not be enlarged, and no
obligation or liability shall arise out of ENCISION’s rendering of technical advice or
assistance in connection with the products sold hereunder.
Limitation of Liability: To the extent allowable by applicable law, in no event
shall ENCISION be liable for any special, incidental or consequential damages in
connection with or arising out of the sale, installation, use, operation, service or
repair of any product, whether based on breach of warranty or contract, strict
liability, negligence or otherwise, whether or not ENCISION shall have been
advised as to the possibility or reason for any such potential loss or damage.
Direct damages shall be strictly limited to the cost to Buyer of the products sold or
provided to Buyer, not withstanding any failure of essential purpose of any limited
remedy.
Any evidence of repair, modication, or resterilization of this product will void
thiswarranty.

AEM EndoShield 19 of 39
Symbol Denitions
Do not Reuse - Single Use Only Sterilized using Ethylene
Oxide
Do not use if the product sterilization
barrier or its packaging is
compromised.
Recycle Battery
Federal (USA) law restricts this
device to sale by or on the order of
aphysician.
Manufacturer
Consult Instructions for Use Temperature Limitation
Catalog Number
non-condensing
Humidity Limitation
Lot Number System Ready
(when illuminated GREEN)
Use by Date Check System
(when illuminated RED)
Refer to Instructions for Use Manufacture Date
HF Isolated Patient Circuit Not made with natural
rubber latex.
Return of Used Product
If for any reason this product must be returned to ENCISION, a returned goods authorization is required
prior to shipping. Appropriate return instructions may be obtained from ENCISION.
Product Changes
ENCISION reserves the right to amend, modify or to change any product, to introduce new products, to
withdraw products and otherwise vary product specications at any time without notice.
US Patents:
# 5,312,401; 5,688,269; 8,007,494; 8,460,284; 8,500,728
ENCISION®, AEM®, and AEM EndoShield® are registered trademarks of ENCISION Inc.
All other referenced trademarks are owned by their respective owners.
Manufactured by:
ENCISION Inc
6797 Winchester Circle
Boulder, CO 80301 USA
Phone: 303.444.2600 Fax: 303.444.2693
Authorized Representative
(according to MDD93/42/EEC)
MDSS GmbH
Schiraben 41 © Copyright 2014 ENCISION Inc.
30175 Hannover, Germany All rights reserved
Printed in USA 05414-004 Rev. E 2016/01
0197

This page left intentionally blank.
Deze pagina is opzettelijk leeg gelaten.
Table of contents
Languages:
Other Encision Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Handicare
Handicare SystemRoMedic ReTurnBelt manual

Mega Electronics
Mega Electronics FemiScan Instruction guide

Weinmann
Weinmann SOMNOvent CR instructions

Skytron
Skytron Skyboom Ergon Series Service manual

THERA-Trainer
THERA-Trainer CORO user manual

Welch Allyn
Welch Allyn 88000A Operating instructions manual