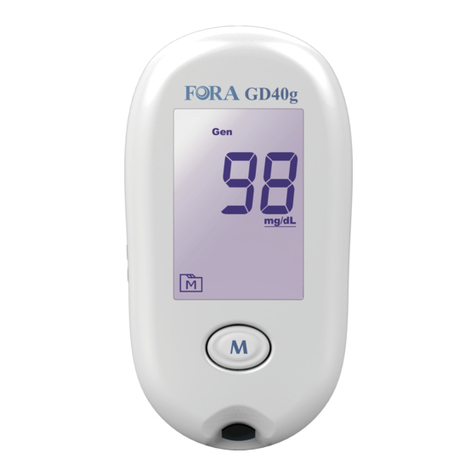
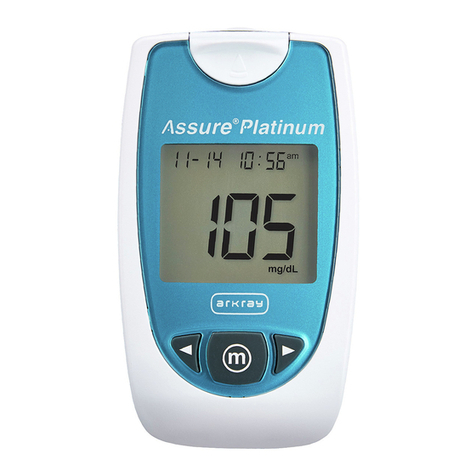
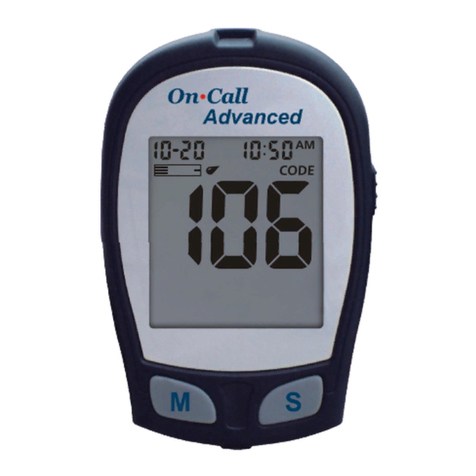
Intended Use
This system is intended for use outside the body
(in vitro diagnostic use) by people with diabetes
at home and by health care professionals
in clinical setting as an aid to monitoring the
effectiveness of diabetes control. It is intended
to be used for the quantitative measurement of
glucose (sugar) in fresh whole blood samples
(from the nger, palm, forearm, upper arm, calf
and thigh). It should not be used for the diagnosis
of diabetes, or testing on newborns.
Professionals may test with capillary and venous
blood sample; home use is limited to capillary
whole blood testing. Venous blood must be
collected only in heparin blood collection tube.
Test Principle
Your system measures the amount of sugar
(glucose) in whole blood. The glucose testing is
based on the measurement of electrical current
generated by the reaction of glucose with the
reagent of the strip. The meter measures the
current, calculates the blood glucose level, and
displays the result. The strength of the current
produced by the reaction depends on the amount
of glucose in the blood sample.
Important Information about
Performing Control Solution
Tests
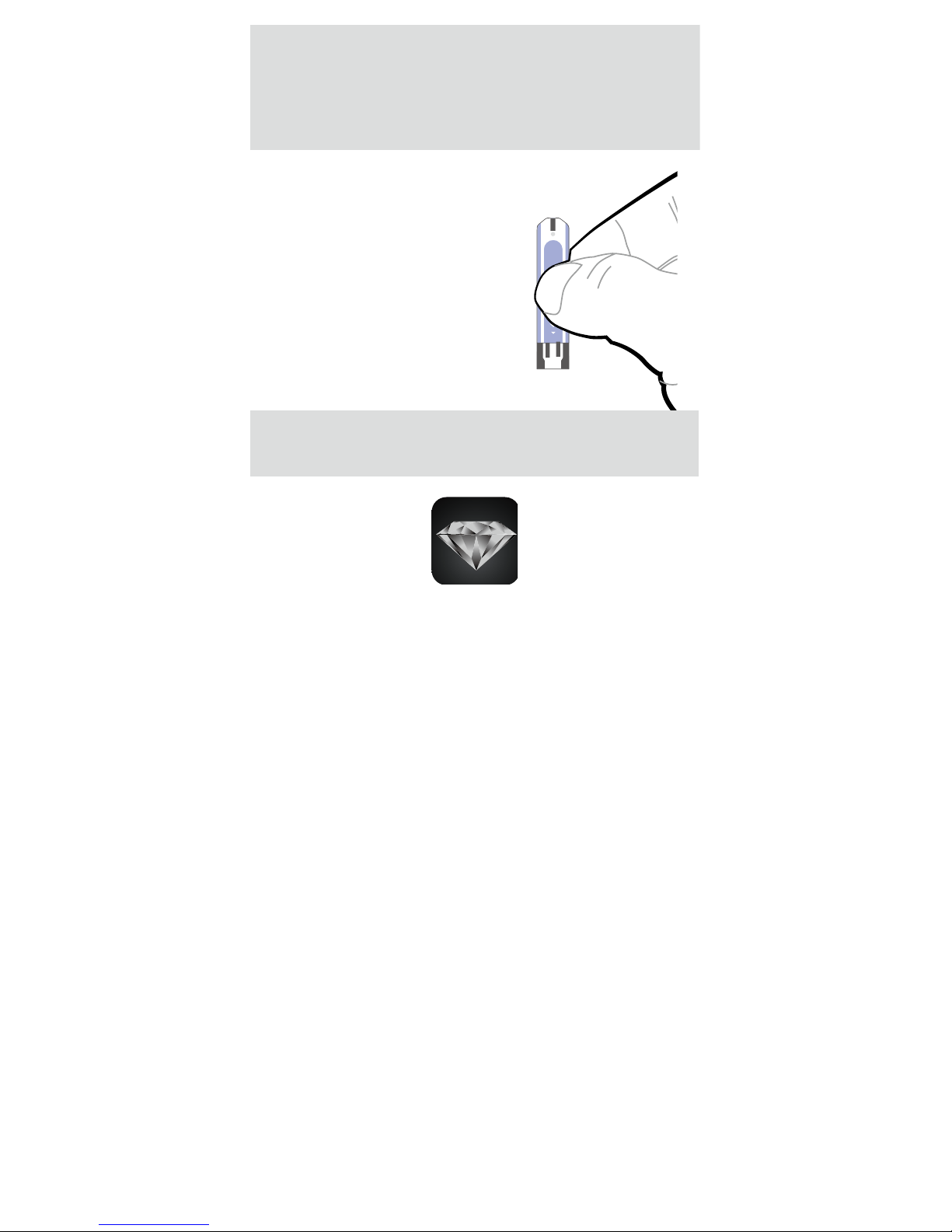
Our Control Solution contains a known amount
of glucose that reacts with test strips and is used
to ensure your meter and test strips are working
together correctly.
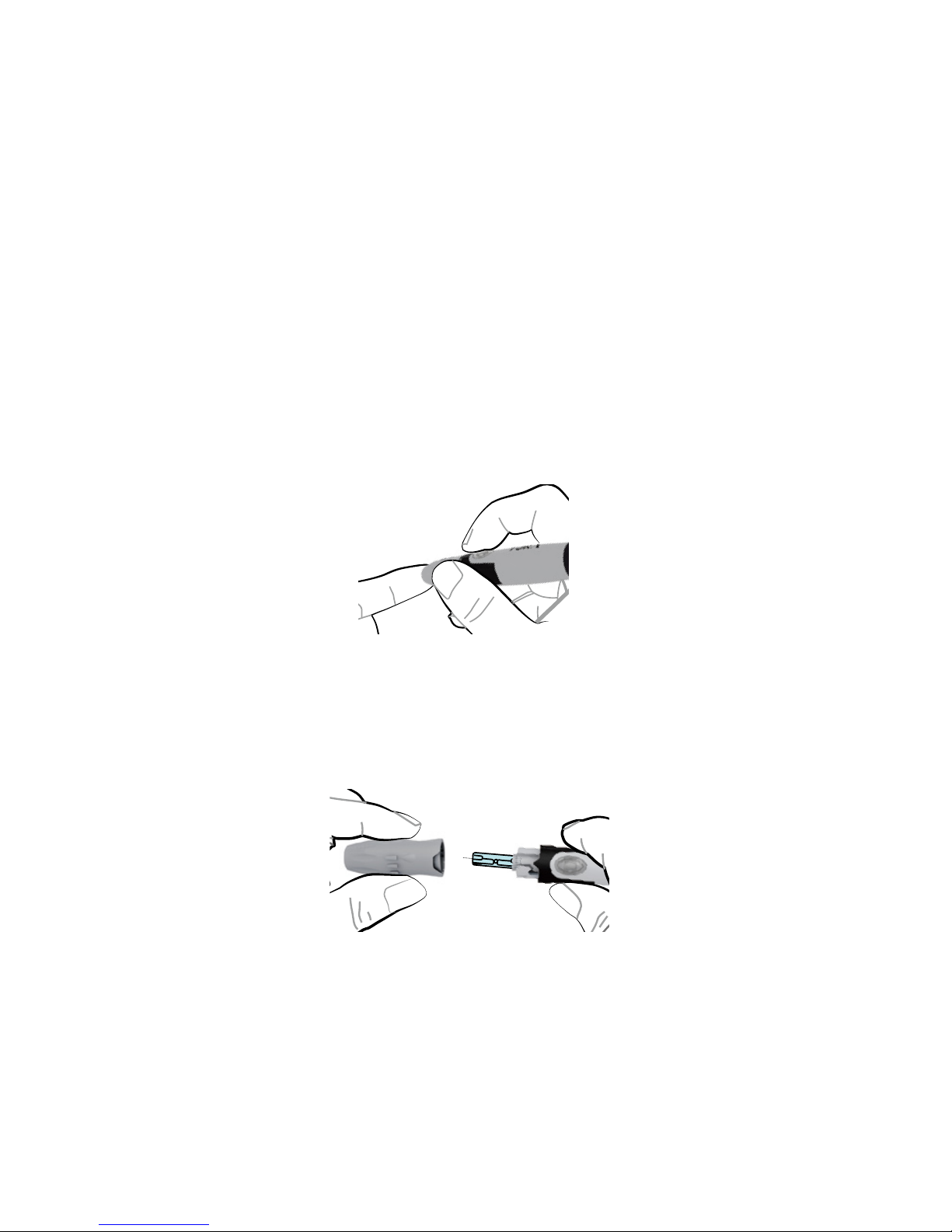
Test strips, control solutions, or sterile lancets
may not be included in the kit (please check
the contents on your product box). They can be
purchased separately. Please make sure you
have those items needed for a blood glucose test
beforehand.
Do a control solution test when:
● you rst receive the meter,
● at least once a week to routinely check the
meter and test strips,