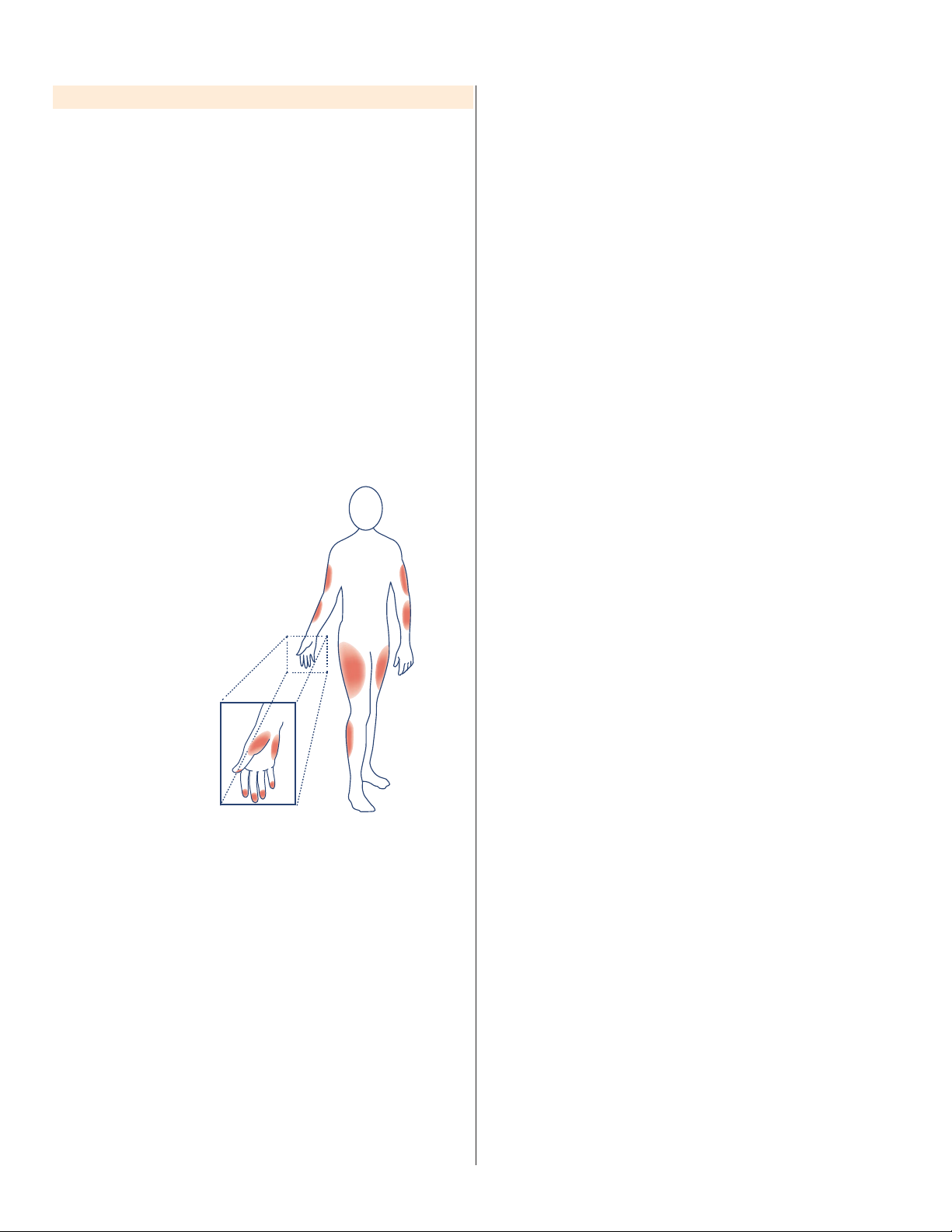
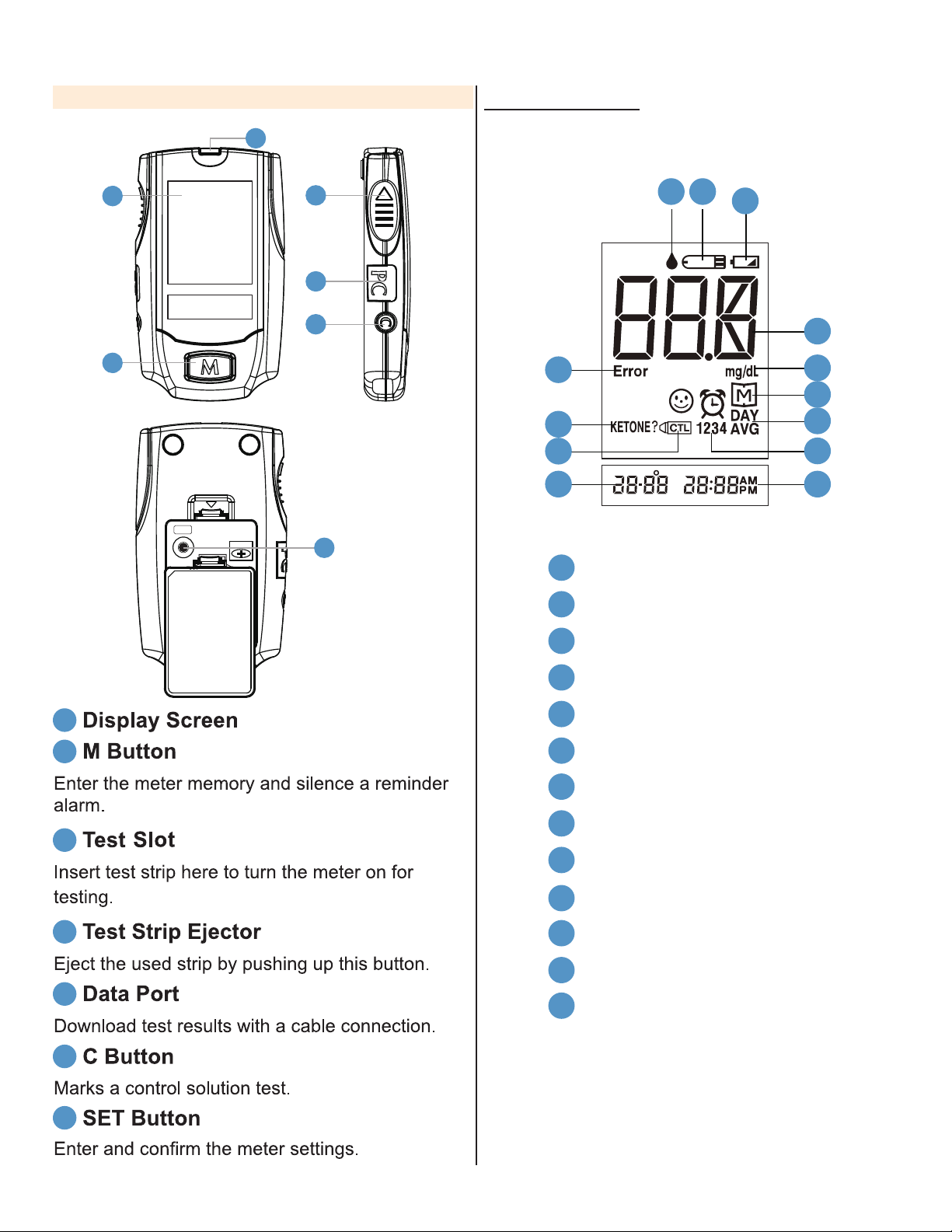
9
display, press Mand select “No” to keep the results
in memory then press SET to skip.
To delete all the results, press Mto select “Yes”.
Then press SET to delete All results. ”OK” is dis-
played in the meter, which means that all data
stored is deleted.
5. Setting the reminder alarm
You may set up any or all of the reminder alarms
(1-4). The meter displays “On” or “OFF” and ,
press Mto turn on or turn off to set the first
reminder alarm.
Press Mto select “On”, then press SET to set the
hour. When the hour is flashing, press Mto add an
hour. Press SET to confirm. Now adjust the
minutes. Press M to add one minute. Hold M
longer to add faster. Press SET to confirm and go
to the next alarm setting.
If you do not want to set an alarm, press SET to
skip this step. If you want to turn off an alarm, find
the alarm number by pressing SET in the setting
mode, press M to change from “ON” to “OFF”. At
the time of your alarm, the meter will beep and
automatically turn on.
You can press Mto silence the alarm and insert
a test strip to begin testing. If you do not press M,
the meter will beep for 2 minutes then switch off.
If you do not want to test at this time, press Mto
switch off the meter.
Quality control testing using the FORA Control
Solution is required to check the performance
of the FORA GD20 Blood Glucose Monitoring
System. The FORA Control Solution checks if the
meter and test strips are working correctly as a
system and if the system is being utilized correctly.
This section explains how to take a control
solution test in order to verify the performance of
FORA GD20 Blood Glucose Monitoring System.
Before testing with the FORA GD20 Blood
Glucose Monitoring System for the first time.
When you open a new vial of test strips.
Whenever you suspect the meter or test strips
may not be functioning properly.
If test results appear to be unusually high or low
or are inconsistent with clinical symptoms.
To check your technique.
When the FORA GD20 meter has been dropped
or you think you may have damaged the meter.
QUALITY CONTROL TESTING
You Should Perform a Control Solution Test
NOTE
These parameters can ONLY be changed in the
setting mode.
If the meter is idle for 3 minutes during the
setting mode, it will automatically switch off.
IMPORTANT:
Depending on different state regulations,
control solution testing may be required on a
daily basis. Refer to your facility procedures for
further details.