User Manual
4| HoverTech International Q2RollerManual Rev. C
Cleaning & Maintenance
CLEANING
The Q2Roller is a single-patient use device. If the device becomes soiled,
completely wipe it down using a germicidal cleaner (phenolic disinfectant,
quaternary solution or other intermediate level disinfectant according to
facility procedure) to disinfect. For best results, follow the cleaner manufac-
turer’s recommended dwell time and instructions for use. Apply germicidal
cleaner directly into hard-to-reach areas. Let air dry. Do not launder.
INFECTION CONTROL
The Q2R Pad is recommended to cover the Q2Roller to keep the device from
getting soiled (also available for separate purchase). Other materials, such as
pads or linens, may also be placed on top of the Q2Roller to keep it clean.
If the Q2Roller is used on an isolation patient, the hospital should employ
the same protocols/procedures it utilizes for the bed mattress and/or for
the linen in that patient room.
PREVENTIVE MAINTENANCE
Prior to use, a visual inspection should be performed on the Q2Roller to
ensure that there is no visible damage that would render the Q2Roller
unusable.
The Q2Roller should be periodically inspected to ensure the following:
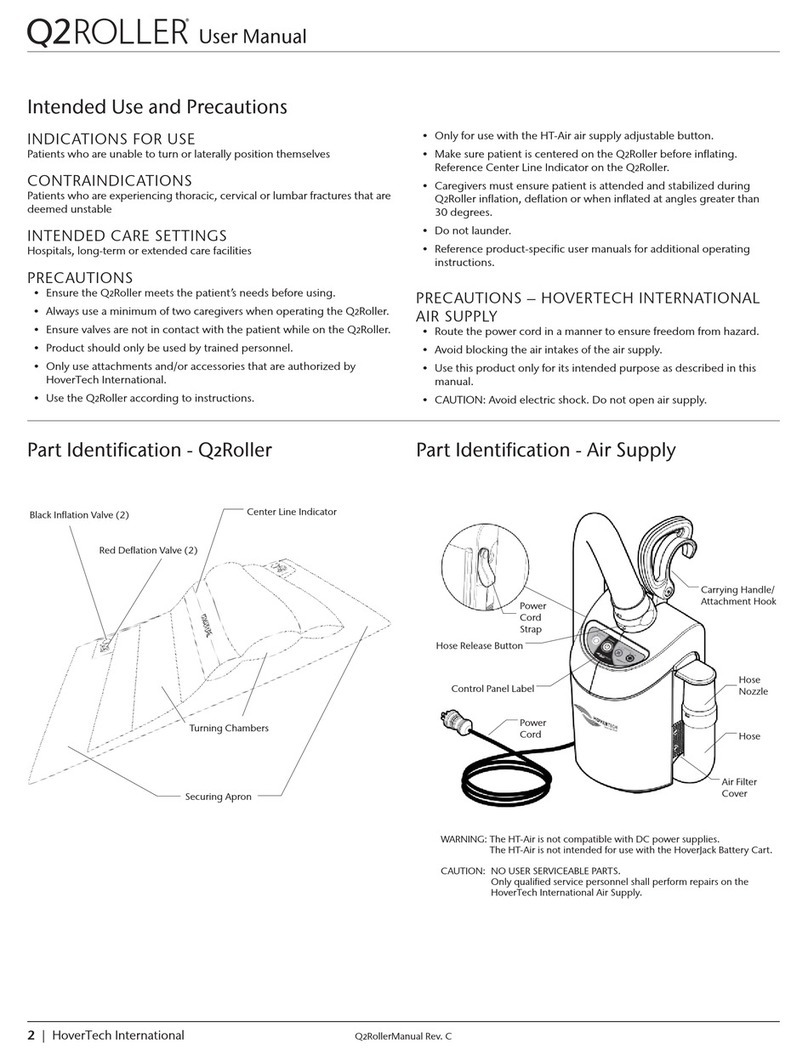
• All inflation valves are self-sealing with no evidence of leakage
• There are no punctures or tears in the Q2Roller
If any damage is found that would cause the Q2Roller not to function as
intended, the Q2Roller should be removed from use.
Warranty Statement
The Q2Roller is warranted to be free from defects in material and workman-
ship. In the unlikely event that a problem arises as a result of a defect in
materials or workmanship, we will promptly replace the Q2Roller within
ninety (90) days of purchase or date of in-service. We will do this at our
expense and discretion using current models or parts performing the
equivalent function upon receipt of the original item to our repair
department. Please refer to the Return and Repairs section of this Manual
for return instructions.
This warranty is not an unconditional guarantee for the life of the product.
Our warranty does not cover product damage that may result from use
contrary to Manufacturer’s instructions or specifications, misuse, abuse,
tampering, or damage due to mishandling. Warranty specifically does not
cover product damage that may result from using an air supply that produc-
es more than 3.5 psi.
Equipment that has been neglected, improperly maintained, repaired or
altered by someone other than an authorized representative of Manufac-
turer, or operated in any way contrary to the operating instructions, shall
void this warranty.
This warranty does not cover normal “wear and tear.” Component parts,
particularly any optional equipment, valve caps, their attachments and
cords, will show wear with use over time and eventually may need to be
refurbished or replaced. This normal type of wear is not covered by our
warranty, but we will provide prompt, high quality repair service and parts
at a nominal cost.
HoverTech International’s liability under this warranty and on any claim of
any kind for any loss or damage arising out of, connected with, or resulting
from the design, manufacture, sale, delivery, installation, repair or opera-
tion of its products, whether in contract or tort, including negligence, shall
not exceed the purchase price paid for the product and upon expiration of
the applicable warranty period, all such liability terminates. The remedies
which this warranty provides are exclusive and HoverTech International
shall not be liable for any incidental or consequential damages.
There are no warranties, express or implied, which extend beyond this
warranty statement. The provisions of these warranty clauses are in lieu of
all other warranties, expressed or implied, and of all other obligations or
liabilities on HoverTech International’s part and neither assumes nor au-
thorizes any other person to assume for HoverTech International any other
liability in connection with Manufacturer sale or lease of said products.
HoverTech International makes no warranties of merchantability or fitness
for a particular purpose. By accepting the goods, the buyer acknowledges
that the goods are suitable for the buyer’s purposes.
MANUFACTURER’S SPECIFICATIONS ARE SUBJECT TO CHANGE.
Returns and Repairs
All products being returned to HoverTech International must have a
Return Goods Authorization Number issued from the company. Please call
800-471-2776 for an RGA #. Any products returned without the necessary
RGA # may cause a delay in the repair time. If the product is not covered
under warranty, a minimum charge of $100 will be assessed for each repair.
Should a repair charge be assessed, HoverTech International will notify the
facility and a purchase order for the repair will need to be issued before the
repair can be completed. Lead-time for repairs is approximately 2 weeks.
All products should be sent to:
HoverTech International
4482 Innovation Way
Allentown, PA 18109
Attn: Repair Dept./RGA #___________________
Phone: 800-471-2776
Fax: 610-694-9601
4482 Innovation Way
Allentown, PA 18109
800.471.2776
Fax 610.694.9601
www.HoverMatt.com
Info@HoverMatt.com