6
INTRAOPERATIVE PROBE
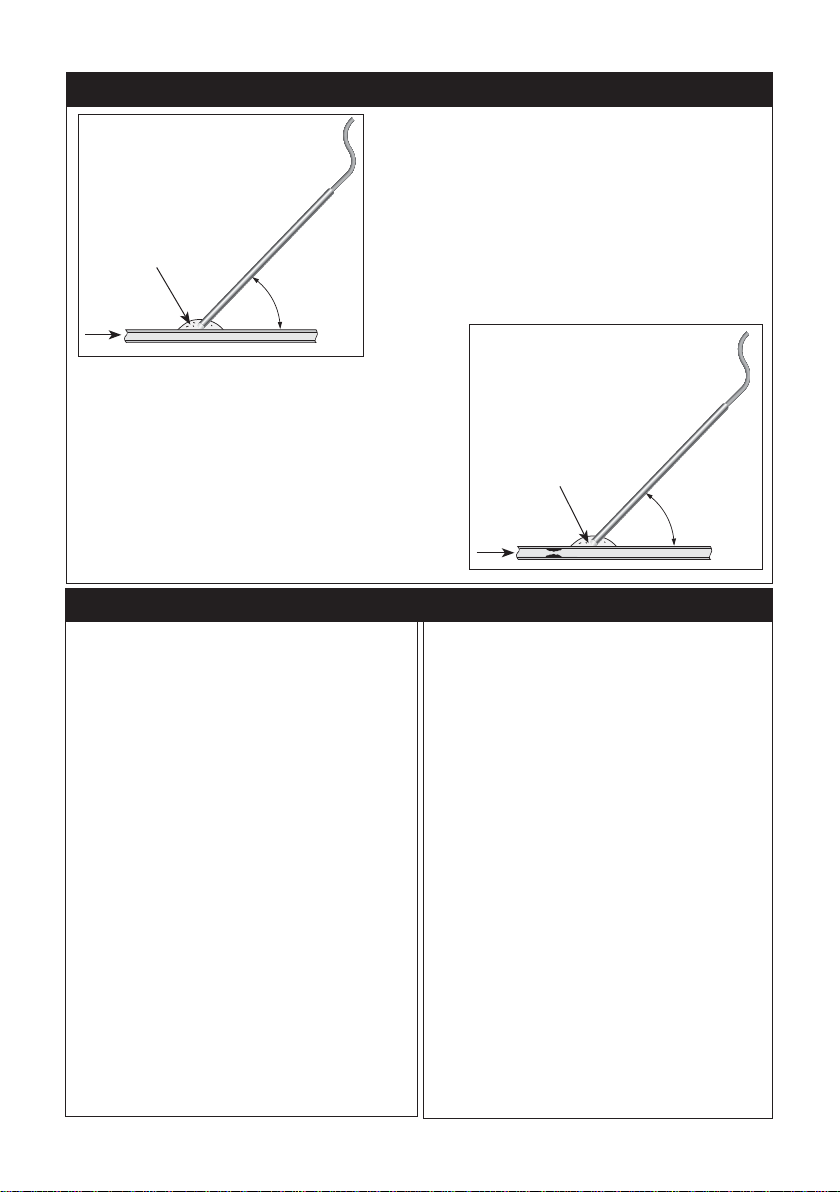
The probe can now be used to assess blood flow in
other vessels maintaining an angle of 45° and ensuring
that the tip is fully wetted. Fig. 2.
Point the probe along the length of the vessel until the
maximum audio signal is heard. The Dopplex DMX unit
will show the bi-directional blood velocity waveform on
its LCD display.
The probe can then be moved along the length of
the vessel, noting any change in pitch of the Doppler
signal or height of the Doppler waveform. This may be
indicative of a change in the lumen area (Fig. 3)
The same procedure can then be carried out after a
graft has been inserted to confirm that adequate blood
flow has been restored. By placing the probe on the
vessel distal to the anastamosis, confirmation of distal
run off is provided.
Fig. 2
Fig. 3
Body Fluid
Body Fluid
45°
45°
WARRANTY & SERVICE
Huntleigh Healthcare‘s standard terms and
conditions apply to all sales. A copy is available on
request.
These contain full details of warranty terms and do
not limit the statutory rights of the consumer.
Service Returns
There are NO USER SERVICEABLE PARTS inside
the probe adaptor or probe.
If for any reason your IOP8 or probe adaptor is
being returned, please:
1. Contact Service Dept. to obtain
authorisation for the product to be returned.
2. Failure to do this may result in the product
being returned without investigation.
3. Clean and sterilise the product, as
described in the cleaning and
sterilisation section.
4. Pack in suitable packing.
5. Attach the decontamination certificate, (or
otherwritten statement declaring that the
product has been cleaned and sterilised),
to the outside of the package.
6. Mark the package “Service Department -
IOP8 Probe/Adaptor
UK
For further details refer to the NHS document
HSG(93)26.
For service, maintenance and any questions
regarding this, or any other Huntleigh Healthcare
Dopplex product, please contact:
Huntleigh Healthcare Ltd
Diagnostic Products Division
35 Portmanmoor Road, Cardiff
CF24 5HN UK
Tel : +44 (0)29 20485885
Fax: +44 (0)29 20492520
Or your local distributor.
Manufactured in the UK by Huntleigh Healthcare.
As part of the ongoing development programme,
the company reserves the right to modify
specifications and materials of the IOP8 and
Probe Adaptor without notice.
Dopplex, Huntleigh and ‘H’ logo are registered
trademarks of Huntleigh Technology Ltd. 2003.
Steris - Steris Corporation, USA
Huntleigh Healthcare 2003