INS0193 JAN 2015 Rev A
MicroSnap Enhanced EB Broth –9ml
Directions for use with MicroSnap EB Detection Device (MS2-EB)
Matrix: Liquid Milk Products
Part No: MS1-EB-BROTH-9ML (100)
Description/Intended Use:
Hygiena’s Enhanced EB Broth is a ready-to-use device compatible with
MicroSnap EB (MS2-EB), MicroSnap Coliform (MS2-COLIFORM) and
MicroSnap E. coli (MS2-ECOLI) detection devices.
Instructions in this insert are for MicroSnap EB (MS2-EB) Detection
Devices with liquid milk samples only.
Enhanced EB Broth contains 9ml of unique liquid medium designed to grow
aerobic and facultative microorganisms while enhancing production of
biomarkers and specific enzymes diagnostic of Enterobacteriaceae,and
reducing sample interferences. The broth has been primarily produced for
liquid milk products. For use with other product samples or incubation times
and temperatures other than 37 ± 0.5°C, please contact Hygiena for
guidance.
Required Materials (Not Provided):
EnSURE luminometer (Part No. ENSURE)
MicroSnap EB Detection Device (MS2-EB)
Dry heating block incubator at 37 ± 0.5°C
Pipettes for transferring 1ml and 0.1ml volumes
Vortex (optional)
Directions:
Instructional Video: www.youtube.com/HygienaTV
Step 1: Enrichment
Enrichment procedure is described below and is alsoshown in Step 1
diagrams.
Visually inspect liquid in tube prior to use. Liquid should be clear and light
straw color, not turbid or cloudy. Use permanent marker to identify sample
on tube label.
1. Allow milk sample and Enhanced EB Broth vial to equilibrate to room
temperature (10 minutes at 22 –26 °C). Unscrew Enhanced EB Broth
cap and aseptically add 1ml liquid milk sample.
2. Replace cap and tighten to secure.
3. Mix vial contents by hand-shaking or vortexing for 10 seconds.
Incubate at 37 ± 0.5°C for 6 to 8 hours, depending on sensitivity
required. Refer to Table 1 below.
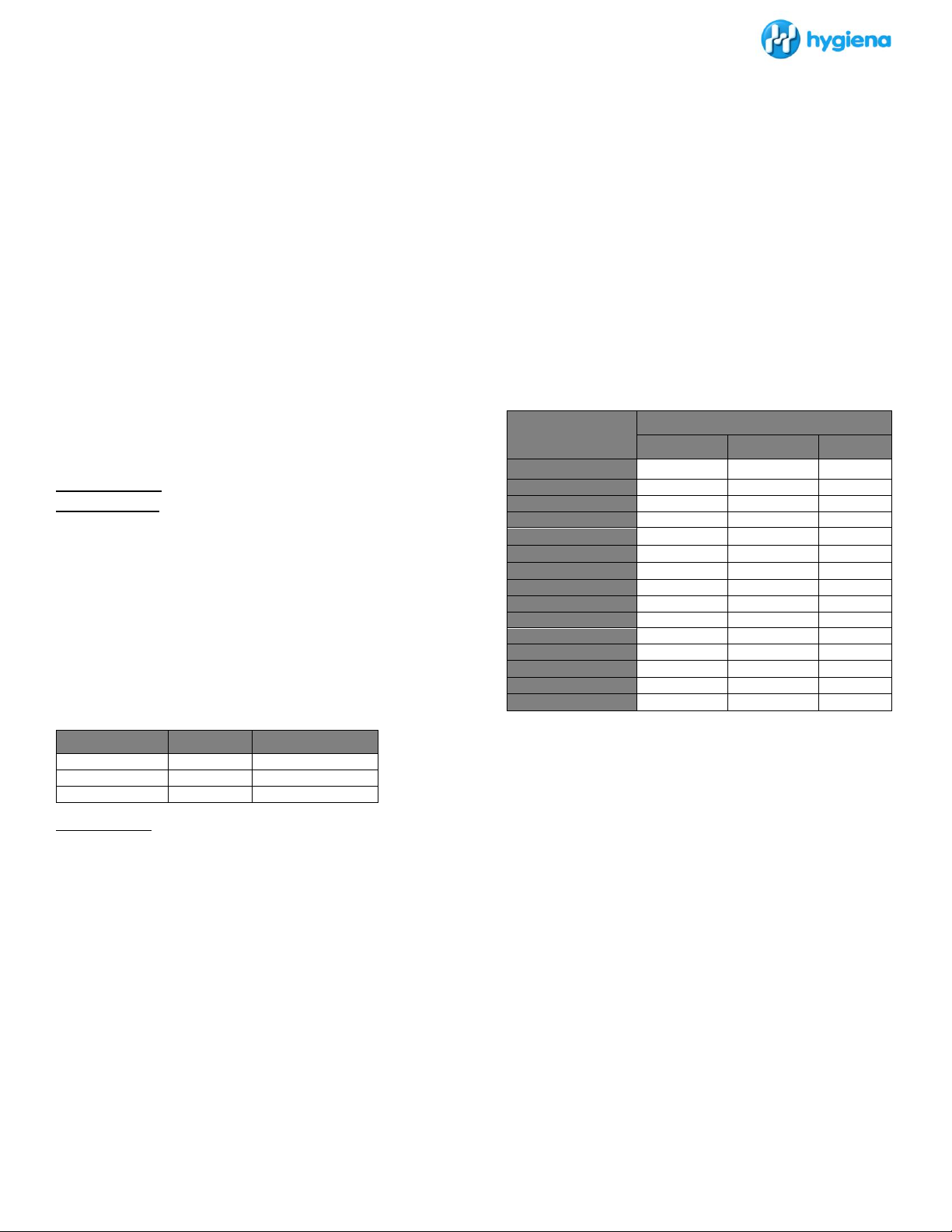
Table 1: Incubation Time & Dynamic Range
Step 2: Detection
Detection procedure is described below and is also shown in Step 2
diagrams.
Turn on luminometer. If locations have been programmed, select location to
be tested.
1. Allow MicroSnap EB Detection Device (Part # MS2-EB) to equilibrate
to room temperature (10 minutes at 22 –26°C). Shake test device by
either tapping on palm of hand 5 times, or forcefully flicking in a
downward motion once. This will bring extractant liquid to bottom of
tube.
2. Remove Enhanced EB Broth tube from incubator andmix by manually
shaking or vortexing for 10 seconds to disperse sample.
3. Open Detection Device by twisting and pulling to remove bulb. Set
aside. Using pipette, aseptically transfer 0.1ml of enriched sample
directly into Detection Device tube.
4. Reassemble Detection Device to original state.
5. Activate Detection Device by holding swab tube firmly and using
thumb and forefinger to break Snap-Valve by bending bulb forward
and backward. Squeeze bulb 3 times to release all liquid to bottom of
swab tube.
6. Shake gently to mix.
7. Immediately insert whole device into luminometer; close lid and
holding unit upright, press “OK” button to initiate measurement.
Results will appear after 15 second count down.
8. Result will be displayed in RLU (relative light units). Set RLU
thresholds on instrument to correspond with required CFU limits.
Refer to “Interpretation of Results” below for correlation.
Interpretation of Results:
Results are displayed as relative light units (RLU). RLU output is
proportional to the starting inoculums and corresponding bacteria
equivalent numbers (expressed as colony forming units, CFU). Table 2
shows equivalent colony forming unit (CFU) values to RLU. This will give an
estimation of the Enterobacteriaceae CFU/mL from the original milk sample.
Table 2: Average relationship between CFU and MicroSnap EB RLU at 6, 7
and 8 hours incubation of 1mL of whole milk naturally contaminated with
organisms at various levels (n=25 per level) incubated at 37 ± 0.5°C
Salmonellae Investigations:
The estimation of Enterobacteriaceae cannot be used as a proxy
measurement for presence or absence of Salmonellae species. For
investigations of Salmonellae presence, a standard method Salmonellae
test should be performed from food or from environmental surfaces.
Calibration & Controls:
It is advisable to run positive and negative controls according to Good
Laboratory Practices. Hygiena offers the following controls:
Calibration Control Kit for Hygiena luminometers (Part # PCD4000)
MicroSnap Coliform & E.coli Positive Controls (Part # MS-PC-
COLIFORM)
Storage & Shelf Life:
Store at 2 –8°C. Devices have a shelf life of 12 months. Check expiration
date on label.
Disposal:
Disinfect before disposal. MicroSnap devices can be disinfected by
autoclaving or by soaking in 20% bleach for 1 hour. Then, they can be
placed in the trash. Alternatively, MicroSnap devices may be discarded at a
biohazard waste disposal facility.