IEM Tel-O-Graph BT User manual

Gebrauchsanweisung
DE
Instructions for Use
EN
Manual de instrucciones
ES
Mode d’emploi
FR
Istruzioni per l'uso
IT
Gebruiksaanwijzing
NL

2
Tel-O-Graph®BT plus
Bloodpressuremonitor
For USA: Caution: Federal law restricts this device to sale by or on the order of a physician
IEM GmbH
Gewerbepark Brand 42
52078 Aachen
Germany
Email:
Website:
www.iem.de
The content of this instructions for use must not be reproduced or published without the written approval of IEM
GmbH.
© IEM GmbH 2021.All rights reserved.

3
EN
Tableof contents
1 Introduction ......................................................... 4
Clinical Validation......................................................5
CE Mark.......................................................................5
Accessories................................................................5
2 Instruction Notes................................................. 6
Intended Use..............................................................6
Improper Use .............................................................6
Essential Performance.............................................7
3 Safety................................................................... 8
Explanation of the safety symbols ........................8
Important patient information ................................9
Important device instructions.............................. 14
4 Description of device ........................................ 17
Blood pressure monitor ........................................ 17
Blood pressure cuff ............................................... 18
Display...................................................................... 19
Ambient conditions................................................ 20
5 Preparing the measurement ............................. 21
Unpacking................................................................ 21
Inserting the batteries ........................................... 22
Switching the blood pressure monitor on/off .. 23
6 Measuring blood pressure and pulse................ 24
Before measuring................................................... 24
Putting the blood pressure cuff on ..................... 24
Correct posture .......................................................28
Measuring ................................................................29
Stopping the measurement..................................30
7 Transferring readings via Bluetooth®................31
Active Pairing (Bluetooth®-Modem)....................31
Passive Pairing (HMS CS).....................................33
8 Memory ..............................................................34
Saving readings ......................................................34
Delete readings from the device ..........................35
9 Cleaning and disinfection ..................................37
Cleaning....................................................................38
Disinfection..............................................................40
10 Maintenance ....................................................41
11 Disposal ...........................................................42
12 Error messages ................................................43
Blood pressure measurement errors ...............43
Communication error ..........................................45
13 Technical data and symbols ............................46
14 Warranty and repairs........................................49
15 Manufacturer’s EMC guidelines.......................51

Introduction
4
1Introduction
Thank you for choosing the Tel-O-Graph®BT upper-arm blood pressure monitor.
Read this operating manual carefully before use and keep it in a suitable place so that the information is available
when required.
The Tel-O-Graph®BT is a fully automated blood pressure and pulse monitor that enables automatic transmission
by means of a Bluetooth®.
The Tel-O-Graph®BT can additionally record the pulse wave form of the pulse, and this information is transmitted
alongside the blood pressure.
A licence key is and the Hypertension Management Software Client Server software (HMS CS) is required to
perform a pulse wave analysis (see HMS CS operating manual). Various forms of pulse wave analysis can be
enabled at any time.
The Tel-O-Graph®BT can be integrated in tele-monitoring systems that may involve different products for data
transmission and storage. Such products, and the data base used to store and assess the blood pressure readings
is not part of the Tel-O-Graph®BT, but is within the responsibility of the care provider/physician that you have
allowed to monitor your blood pressure readings. You may not have direct access to the database, and need to
contact the care provider/physician, if you have any question related to the stored data.
This operating manual explains the blood pressure monitor and accessories in the order in which you will operate
the monitor and also use later.
If you have any questions about our services or products, feel free to contact us.
Pulse Wave Analysis (PWA) is not available in the USA.

Introduction
5
DE
EN
Clinical Validation
The accuracy of the device's measurements has been validated in accordance with ISO 81060-2:2013.
CE Mark
The Tel-O-Graph®BT meets the requirements of the
▪93/42/EEC (MDD),
▪2014/53/EU (RED),
▪2011/65/EU (RoHS) guidelines
and bears the CE mark.
IEM GmbH hereby declares that the Tel-O-Graph®BT corresponds to the 2014/53/EU (RED) guideline.
The complete text of the EU declaration of conformity is available at the following website address:
https://www.iem.de/doc/
Accessories
Medical accessories
▪Blood pressure cuff "S" (Arm circumference:20-24 cm (7.9-9,5 in))
▪Blood pressure cuff "M" (Arm circumference: 24-32 cm (9.5-12.6 in))
▪Blood pressure cuff "L" (Arm circumference: 32-38 cm (12.6-15.0 in))
▪Blood pressure cuff "XL" (Arm circumference: 38-55 cm (15.0-21.7 in)
▪HMS CS
General accessories
▪Batteries (4x, AA, alkaline)
▪USB-Bluetooth®-Adapter
▪IEM set pouch

Instruction Notes
6
2Instruction Notes
Intended Use
The Tel-O-Graph®BT is intended for the measurement of blood pressure and pulse on the upper arm in adults.
The blood pressure monitor is suitable for individuals with an arm circumference of 20-55 cm (7.9-21.7 in) when
used with the corresponding monitor cuff size.
The data measured is automatically transmitted.
The Tel-O-Graph®BT with PWA-license additionally records pulse waveform data.
Pulse Wave Analysis (PWA) is not available in the USA.
WARNING
Self-diagnosis and self-treatment on the basis of the results is dangerous!
▪Do not undertake any treatment and/or take medication as a result of the measured values without
consulting your physician.
▪Follow your physician‘s instructions.
Improper Use
The blood pressure measuring device must not be used for newborn infants or children under the age of 12, must
not be used for surgery, must not be used near a magnetic resonance imaging scanner or other strong magnetic
field, and must not be used for monitoring patients within a clinical context or during their transport.
The blood pressure measuring device must be kept out of the reach of unsupervised children and must not be used
on people deemed legally incompetent.
It must not be used for any other purpose than the blood pressure measurement procedure described here and
must not be used in vehicles or aircraft!

Instruction Notes
7
DE
EN
The Tel-O-Graph®BT is not designed to be used on pregnant women or in cases of pre-eclampsia.
Note
▪There are currently no clinical studies available on the use of pulse wave analysis in children. Accordingly,
there is no confidence interval for persons under the age of 20 years.
▪If you are taking medication to alter blood clotting, consult your physician before using the blood pressure
monitor.
Essential Performance
The essential performance features are defined as blood pressure measurement with:
▪Error tolerances of the pressure gauge and measurement results within the required limits according to IEC
80601-2-30.
▪Maximum change value in blood pressure determination according to IEC 80601-2-30.
▪Power delivery (pressure supply to the cuff) within the set limits according to IEC 80601-2-30.
▪An error is issued in the event that successful blood pressure measurement is impossible.
The blood pressure monitor does not emit an alarm in the sense of IEC 60601-1-8. The blood pressure monitor is
not provided to be used in conjunction with RF surgery monitors or for the clinical monitoring of patients, such as
on an intensive care unit.
Basic safety means that the patient cannot be endangered by any automatic device procedure.
In the event of an unclear status or state of the blood pressure monitor, the blood pressure monitor must enter
standby mode by the device releasing the air in the cuff. The cuff is not automatically pressurized, to do so, the
device must be initiated manually.

Safety
8
3Safety
This section explains all the safety information for the device.
Read this section carefully before using the blood pressure monitor.
Contact your physician before using the device if you are pregnant, are taking medication to alter blood clotting or
if you have been diagnosed with cardiac arrhythmia, coagulation disorders or arteriosclerosis.
Explanation of the safety symbols
WARNING
Short description of the danger
This warning symbol in connection with the signal word WARNING indicates a possible or immediately
threatening danger.
Non-adherence may lead to the mild, moderate injuries or to the most severe injuries or death.
ATTENTION
Short description of the danger
This warning symbol, in connection with the signal word ATTENTION, indicates possible material damage.
Non-adherence may lead to damage to the products or their accessories.

Safety
9
DE
EN
Note
The signal word Note indicates further information about the Tel-O-Graph®BT or its accessories.
External Reference
Indicates reference to external documents in which further information may optionally be found.
Important patient information
WARNING
Danger as a result of self-diagnosis
▪Do not undertake any changes to your treatment and/or take medication due to the measured values
without consulting your physician.
▪Follow your physician‘s instructions.
WARNING
Danger of blood flow disruptions as a result of putting on and pumping up a cuff on limbs with an intra-
vascular drip or intra-vascular treatment or with an arteriovenous (AV) shunt.
▪If you have an intra-vascular drip or arteriovenous (AV) shunt in one of your arms do not place the .cuff
at this arm.

Safety
10
WARNING
Danger of tissue bleeding or haematoma.
▪When using the blood pressure monitor, make sure it does not impede the blood circulation in your arm.
▪If you have sensitive bodily tissue, despite the correct positioning of the cuff, it can still result in tissue
bleeding or haematoma.
▪If you are taking medication to alter blood clotting or suffer from coagulation disorders, consult your
physician before using the blood pressure monitor.
WARNING
Danger of injury as a result of allergic reactions to the cuff material
▪In the event of pain or allergic reactions, remove the cuff.
▪Pay attention to hygiene concerns.
WARNING
Danger of injury as a result of using unapproved accessories
▪Only use accessories approved by the manufacturer and distributed by the trader or manufacturer.
▪Read the respective information provided by the manufacturer before using the accessories for the first
time.
▪Before use, check accessories in relation to the manufacturer specifications.

Safety
11
DE
EN
WARNING
Danger of injury as a result of putting on or pumping up a cuff on an arm on the same side of the body as a
mastectomy has been carried out
▪Do not apply the Tel-O-Graph®BT cuff to the arm on the side where a mastectomy was performed.
WARNING
Danger of a temporary loss of function of a present electrical medical device as a result of putting on or
pumping up a cuff if you are wearing a further electrical medical device for monitoring on the same limb.
▪Only apply the Tel-O-Graph®BT cuff if you are not wearing any other medical electrical device on this
arm.
WARNING
Danger of fluid occurrence when using the batteries incorrectly
▪Liquid that escapes from the batteries due to mishandling can cause skin irritation. If you come into
contact with the liquid, rinse it away with plenty of water. If the liquid comes into contact with your eyes,
do not rub your eyes but instead immediately rinse them with water for 10 minutes and contact a
physician without delay.

Safety
12
WARNING
Danger of blood flow interruptions as a result of steady cuff pressure or too frequent measuring
▪Ensure the cuff hose is in the correct position and take care that the cuff hose is not knotted, pinched,
kinked or stretched.
▪If you notice pain, swelling, reddening or numbness in your arm, around which the cuff is placed, inform
your physician. (It is expected that some mild to moderate discomfort may be experienced during a
blood pressure measurement.)
▪Measurement can be interrupted at any stage by pressing the button. This deflates the cuff
and the device can be removed.
WARNING
Danger of strangulation by the cuff hose
▪Persons (including children) who are unable to use the blood pressure monitor safely due to their
physical, sensory or mental capabilities or their inexperience or lack of knowledge must not use this
blood pressure monitor without supervision or instruction by a responsible person.
▪The blood pressure monitor may not be used by those with limited mental competencies. (Keep out of
reach.)
▪Do not wrap the cuff or the cuff hose around your neck!
▪The cuff must only be worn on the upper arm!
▪Check the correct positioning of the cuff.
▪If you notice pain, swelling, reddening or numbness in the arm around which the cuff is placed, inform
your physician. (It is expected that some mild to moderate discomfort may be experienced during a
blood pressure measurement.)

Safety
13
DE
EN
▪Measurement can be interrupted at any stage by pressing the button. This deflates the cuff and the cuff
can be removed.
WARNING
Risk of injury if used on patient groups for whom this device is not intended
▪The Tel-Graph® BT is not intended for use on women who are pregnant or those with pre-eclampsia.

Safety
14
Important device instructions
ATTENTION
Equipment failure
▪The device must not be used in the vicinity of magnetic resonance imaging apparatus or in the direct
proximity of another electrical medical monitor.
▪The device is not suitable for simultaneous use with high frequency surgery monitors.
▪Do not drop the blood pressure monitor and do not place objects on top of it.
▪Do not use the device directly adjacent to other devices or stacked with other devices, as this may result
in malfunction. If operation in the manner described above becomes necessary nevertheless, this device
and the other devices should be monitored to ensure that they are functioning correctly
▪Use of components other than those supplied with the device may result in measurement errors, as
other equipment (e.g. transformers and cables) may cause increased electromagnetic interference or
have reduced electromagnetic immunity. You should therefore only use genuine IEM accessories.
▪The cuff and the hose are made of a material that does not conduct electricity. They thus protect the
device against the effects of discharging a defibrillator. In the event of discharging a defibrillator, the
device itself must not touch the patient since the device can be damaged as a result of such a
discharging and can result in the incorrect value being displayed.
ATTENTION
Warranty
▪Do not open the housing of the Tel-O-Graph®BT, otherwise any warranty becomes void.

Safety
15
DE
EN
ATTENTION
Batteries
▪Remove the batteries from the battery compartment when they no longer have any charge or if you do
not expect to use the blood pressure monitor for a longer period of time.
▪Do not throw batteries into fire and never expose them to high temperatures!
▪Do not attempt to recharge the batteries. Do not attempt to open or short-circuit the batteries. There is a
risk of explosion.
ATTENTION
Electric fields
▪Measurements may be faulty if the device is operated in the vicinity of strong electrical fields. Do not
operate the blood pressure monitor near:
oHigh-voltage power lines
oMicrowave devices
▪Portable and mobile RF transmitters, such as mobile phones for example, may affect the blood pressure
monitor. Transmission of data via mobile communication networks may be disrupted by other devices,
even if those devices comply with the applicable transmission requirements specified by CISPR. You
should therefore ensure that the Tel-O-Graph®BT is at least 30 cm (12 inches) from any portable RF
communications equipment.

Safety
16
ATTENTION
Fluid damage to the blood pressure monitor
▪Liquid must not penetrate the device. If you believe that liquid has penetrated the device during cleaning
or use of the blood pressure monitor, the device must no longer be used.
▪If the blood pressure monitor is exposed to moisture, switch the blood pressure monitor off and remove
the batteries. Immediately inform your healthcare provider/physician.
Note
This blood pressure monitor is intended for use in home healthcare environments and professional healthcare
institutions, such as first aid facilities and hospitals.
Description of device
17
DE
EN
4Description of device
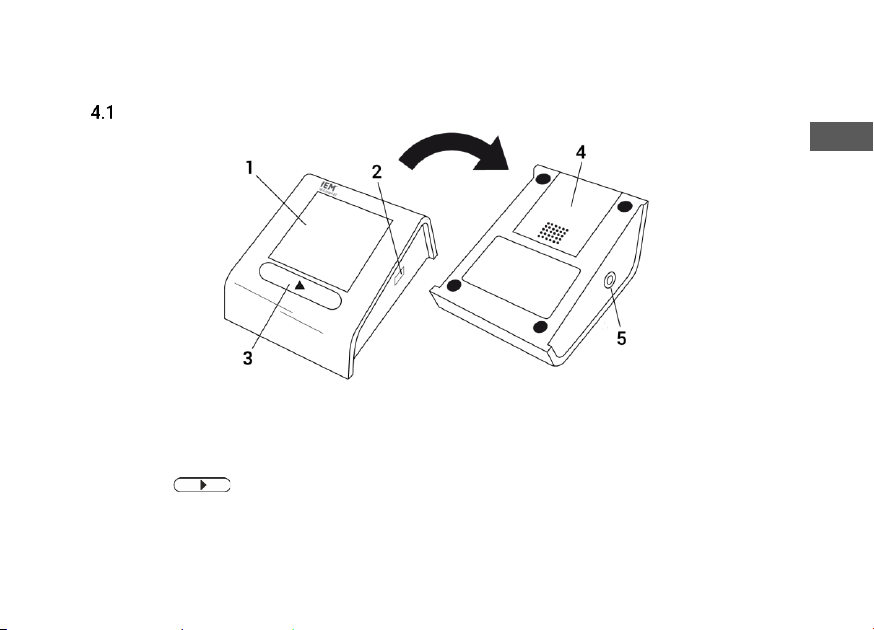
Blood pressure monitor
Fig. 1: Blood pressure monitor
1. Display
2. Infrared interface (for service)
3. - Button
4. Battery cover
5. Air hose socket

Description of device
18
Blood pressure cuff
Fig. 2: Blood pressure cuff
1. Blood pressure cuff
2. Air hose
3. Air hose connection
Description of device
19
DE
EN
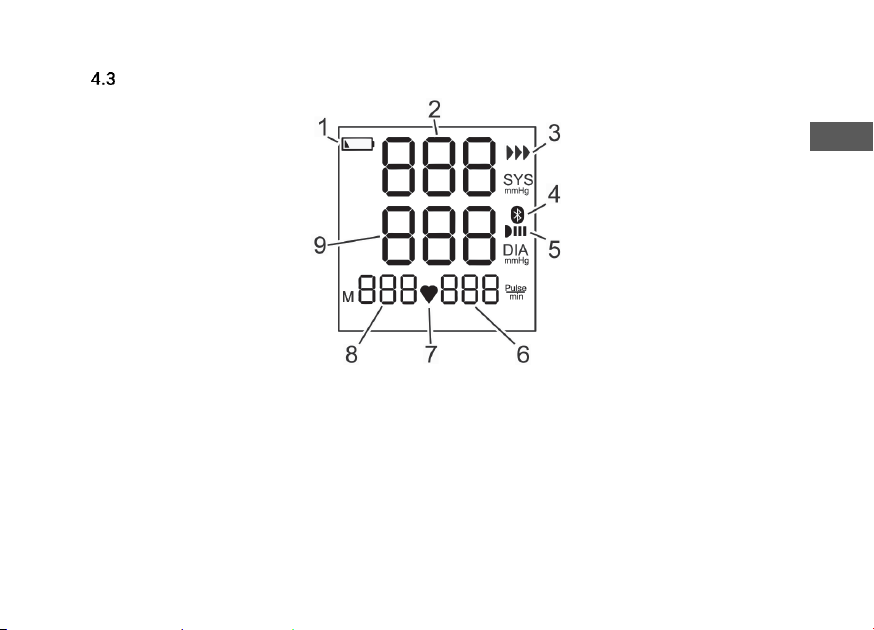
Display
Fig. 3: Display
1. When this appears: Battery empty
2. Display of systolic (upper) value
3. Data transfer
4. Bluetooth®
5. Infrared communication
6. Number of pulse beats per minutes
7. Pulse detected
8. Number of measurement values
9. Display of diastolic (lower) value

Description of device
20
Ambient conditions
ATTENTION
▪Extreme temperatures, humidity or air pressure can influence measurement accuracy. Please follow the
operating instructions.
▪Extreme temperatures, humidity or altitude can affect the performance of the blood pressure monitor.
Do not store the device near a fireplace or heating unit and do not expose it to intense sunlight. Do not
place the device near a nebuliser or steam generator, as the condensation may damage it.
▪Never store the blood pressure monitor outside a temperature range of -25 °C to +70 °C.
▪Never use the blood pressure monitor outside a temperature range of +5 °C to +40 °C.
▪Only store or use the blood pressure monitor at a relative air humidity (not condensing) of 15 % to 93 %.
▪The blood pressure monitor takes approx. 25 minutes to go from the minimum storage temperature of
-25 °C to the operating temperature of +5 °C in an ambient temperature of +20 °C.
▪The blood pressure monitor takes approx. 25 minutes to go from the maximum storage temperature of
+70 °C to the operating temperature of +40 °C in an ambient temperature of +20 °C.
Other manuals for Tel-O-Graph BT
2
Table of contents
Other IEM Blood Pressure Monitor manuals

IEM
IEM Mobil-O-Graph PWA User manual

IEM
IEM Mobil-O-Graph NG User manual

IEM
IEM agedio B500 User manual

IEM
IEM Tel-O-Graph GSM User manual

IEM
IEM agedio B500 User manual
IEM
IEM Tel-O-Graph BT User manual

IEM
IEM Tel-O-Graph BT User manual

IEM
IEM Mobil-O-Graph NG User manual

IEM
IEM Mobil-O-Graph NG User manual

IEM
IEM Tel-O-Graph GSM User manual