1
Table Of Contents
Introduction / About This Manual.................................................................................................................. 3
Preparation For Use....................................................................................................................................... 3
Unpack The Unit ......................................................................................................................................... 3
Initial Inspection .......................................................................................................................................... 3
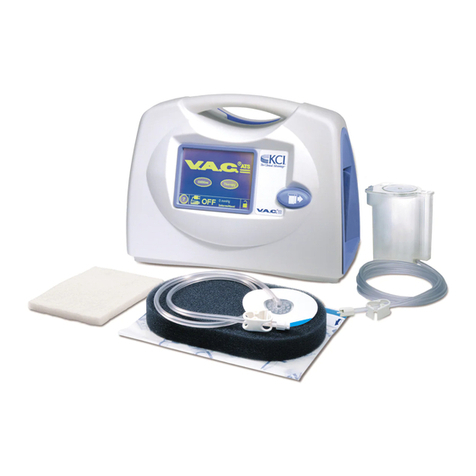
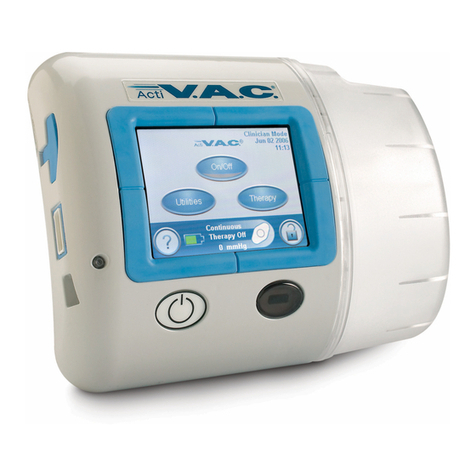
Unit Features ................................................................................................................................................ 4
Serial Number Location ............................................................................................................................... 5
Cleaning and Disinfection ............................................................................................................................. 5
Infection Control......................................................................................................................................... 5
Supplies and Equipment Needed ................................................................................................................. 5
General Cleaning Recommendations........................................................................................................... 5
Therapy Unit ............................................................................................................................................... 6
Power Supply .............................................................................................................................................. 7
Fabric Carrying Case ................................................................................................................................... 7
Service Procedures ........................................................................................................................................ 8
Parts and Equipment Needed ...................................................................................................................... 8
Inspect Unit for Damage ............................................................................................................................. 8
Replace Exhaust Filter.................................................................................................................................. 9
Data Download........................................................................................................................................... 9
Battery Check ........................................................................................................................................... 10
Battery Change ......................................................................................................................................... 11
Pressure Checks ........................................................................................................................................ 13
• Set-up ................................................................................................................................................. 13
• 25 mmHg Test ..................................................................................................................................... 14
• 125 mmHg Test ................................................................................................................................... 14
• 200 mmHg Test ................................................................................................................................... 15
• 125 mmHg Test .................................................................................................................................. 16
Charge Battery and 6 Hour Unit Verification Test....................................................................................... 17
Verify Time and Date................................................................................................................................. 20
Alarm Tests ............................................................................................................................................... 20
• Leak Alarm .......................................................................................................................................... 20
• Blockage Alert ..................................................................................................................................... 21
• Canister Not Engaged and Check Battery Level.................................................................................... 22
• Canister Full Therapy Interrupted ......................................................................................................... 22
Final Settings............................................................................................................................................. 23
Recharge Battery....................................................................................................................................... 23
Preparation for Transport and Patient Use ................................................................................................... 24
On-Premises Use ....................................................................................................................................... 24
• Supplies needed: ................................................................................................................................. 24
• Preparation For Use ............................................................................................................................. 24
Off-Premises Use....................................................................................................................................... 25
• Supplies needed: ................................................................................................................................. 25
• Preparation For Use ............................................................................................................................. 25
Specifications.............................................................................................................................................. 26
Spare Parts ................................................................................................................................................. 27
Symbols Used ............................................................................................................................................. 28
Service Manual.......................................................................................................................................... 28
Therapy Unit ............................................................................................................................................. 28
Contact Information ................................................................................................................................... 28
ActiV.A.C.®Therapy System Required Service Record............................................................ Inside Back Cover