1
Table Of Contents
WARNING.......................................................................................................................................................................................................... 3
Important Safety Information Accompanies This Device............................................................................................................. 3
DISCLAIMER OF WARRANTY AND LIMITATION OF LIABILITY......................................................................................................... 3
Important Information For Users............................................................................................................................................................. 4
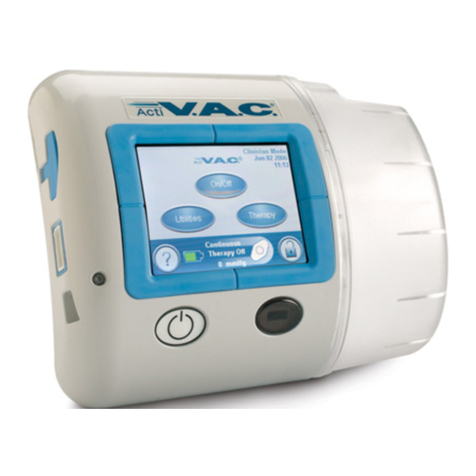
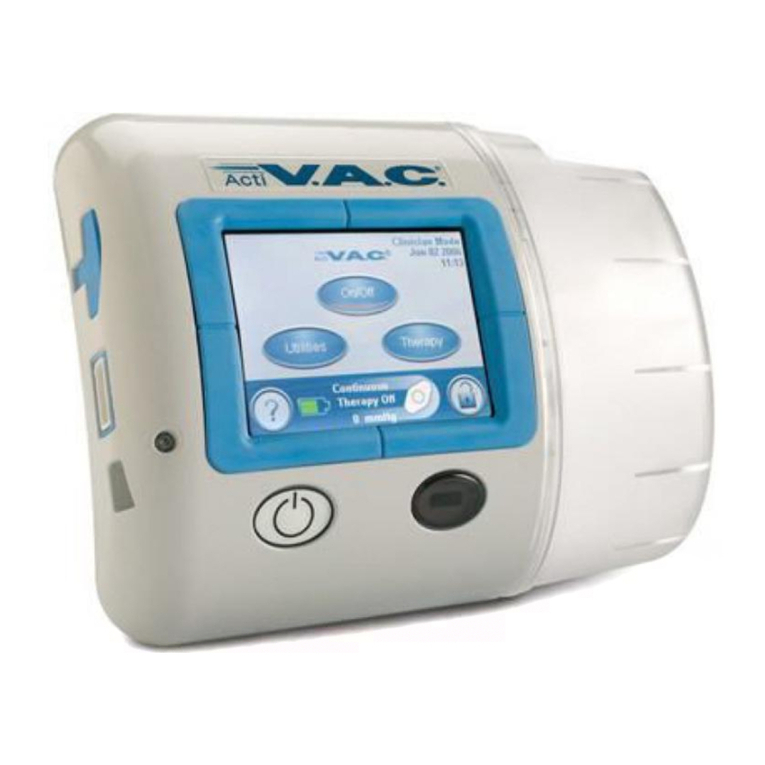
InfoV.A.C.® Therapy Unit Feature Identification.................................................................................................................................. 5
Introduction / About This Manual........................................................................................................................................................... 6
Preparation For Use ...................................................................................................................................................................................... 6
Unpack the Unit........................................................................................................................................................................................... 6
Initial Inspection.......................................................................................................................................................................................... 6
Serial Number Location............................................................................................................................................................................ 7
Cleaning and Disinfection.......................................................................................................................................................................... 7
Infection Control ......................................................................................................................................................................................... 7
Supplies and Equipment Needed......................................................................................................................................................... 7
General Cleaning Recommendations.................................................................................................................................................. 7
Therapy Unit ................................................................................................................................................................................................. 8
Power Supply................................................................................................................................................................................................ 9
Service Procedures .....................................................................................................................................................................................10
Inspect Unit For Damage .......................................................................................................................................................................10
•Hanger Arm and Rubber Anti-slip Pad Inspection................................................................................................................12
Battery Check / Change..........................................................................................................................................................................13
Charge Battery ...........................................................................................................................................................................................15
Power On / Screen Inspection..............................................................................................................................................................16
Data Transfer...............................................................................................................................................................................................16
Canister Bellows Check...........................................................................................................................................................................17
Canister Fit...................................................................................................................................................................................................18
Verify Time and Date................................................................................................................................................................................19
Testing Procedures......................................................................................................................................................................................20
Pressure Transducer Check....................................................................................................................................................................20
•Test Canister Preparation................................................................................................................................................................20
•Unit Preparation.................................................................................................................................................................................21
•Engineering Screens Access ..........................................................................................................................................................21
•Sensor Accuracy Test........................................................................................................................................................................22
•Zero Pressures.....................................................................................................................................................................................22
Pressure Checks.........................................................................................................................................................................................23
•Test Canister Preparation................................................................................................................................................................23
•Unit Preparation.................................................................................................................................................................................23
•Test Procedure....................................................................................................................................................................................23
Alarm Tests ..................................................................................................................................................................................................25
•Canister Preparation.........................................................................................................................................................................25
•Unit Setup ............................................................................................................................................................................................26
•Leak Alarm ...........................................................................................................................................................................................26
•Blockage Alert ....................................................................................................................................................................................26
•Canister Not Engaged......................................................................................................................................................................27
•Canister Full Therapy Interrupted................................................................................................................................................27
Testing Complete......................................................................................................................................................................................27
Final Settings ..............................................................................................................................................................................................28
Recharge Battery.......................................................................................................................................................................................28