7
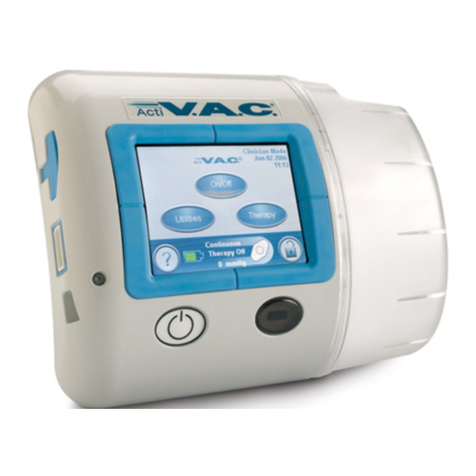
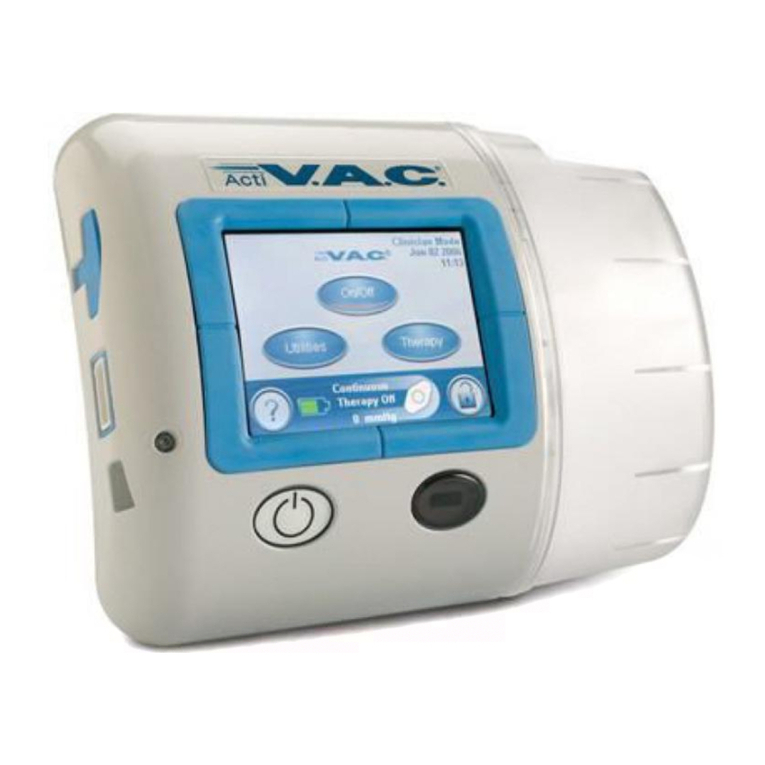
PREVENA PLUS™ 125 Therapy Unit
Explanation of Symbols Used
Do not use if package is damaged or open Rx only CAUTION: Federal (US) law restricts this
device to sale by or on the order of a
physician.
IP22 Ingress Protection Consult Instructions for Use
Type BF applied part Refer to Clinician Guide
Sterile using radiation Single Use Only
Date of Manufacture Contains Phthalates
(SENSAT.R.A.C.™ Pad Tubing)
Manufacturer Tripping Hazard
Fragile Use By
Keep Dry Catalog Number
Content Information Lot Number
Class II Device
2
STERILE
Do Not Resterilize
Temperature Limit
This product is designated for separate
collection at an appropriate collection
point. Do not dispose of as household
waste.
MR Unsafe No Bathing or Showering
Electrical Safety/Electromagnetic Compatibility Information
Electrical Safety
• Do not operate this product if it has a damaged power cord, power supply or plug. If these
components are worn or damaged, contact KCI.
• The PREVENA PLUS™ Incision Management System is classified as a Type BF applied part
under IEC 60601-1.
• It provides IP22-Protection level against ingress of solid foreign objects and liquids.
• All alerts are classified as low priority according to IEC 60601-1.
• Conforms to: IEC 60601-1, IEC 60601-1-6, IEC 60601-1-11, IEC 60601-1-8.
Electromagnetic Compatibility
The PREVENA PLUS™ 125 Therapy Unit needs special precautions regarding EMC.
WARNING: As with all electrical medical equipment, the therapy unit may cause radio
interference or may disrupt the operation of nearby equipment. It may be necessary to
take mitigation measures such as re-orienting or relocating the PREVENA PLUS™ 125
Therapy Unit or shielding the location.
• Portable and Mobile RF communications equipment including cell phones and similar
devices, RFID readers, electronic article surveillance (anti-theft) equipment and metal
detectors can affect the performance of the PREVENA PLUS™ 125 Therapy Unit.
• Other medical equipment or systems can produce electromagnetic emissions and
therefore can interfere with the functionality of the PREVENA PLUS™ 125 Therapy Unit.
Care should be used when operating the PREVENA PLUS™ 125 Therapy Unit adjacent to or
stacked with other equipment. If adjacent or stacked use is necessary, the PREVENA PLUS™
125 Therapy Unit and the other equipment should initially be observed to verify normal
operation in the configuration in which it will be used.
• The electrical cable, external power supply and accessories provided by the manufacturer
have been shown to comply with the test requirements. Use only the manufacturer-
supplied electrical cable, power supply and accessories with the PREVENA PLUS™ 125
Therapy Unit.
• Portable and mobile RF communications equipment (including peripherals such as
antenna cables and external antennas) should be used no closer than 30 cm (12 inches)
to any part of the PREVENA PLUS™ 125 Therapy Unit including power cord and power
supply provided by the manufacturer. Otherwise, degradation of the performance of this
equipment could result.
• NOTE: This equipment has been tested and found to comply with the limits for medical
devices to IEC 60601-1-2: 2014 4th edition. These limits and test levels are intended to
provide reasonable safety with regard to electromagnetic disturbances when the device is
used in a typical medical installation.