LMW Microscopy Lab : Leica SP2 Page 9 `6/26/2012
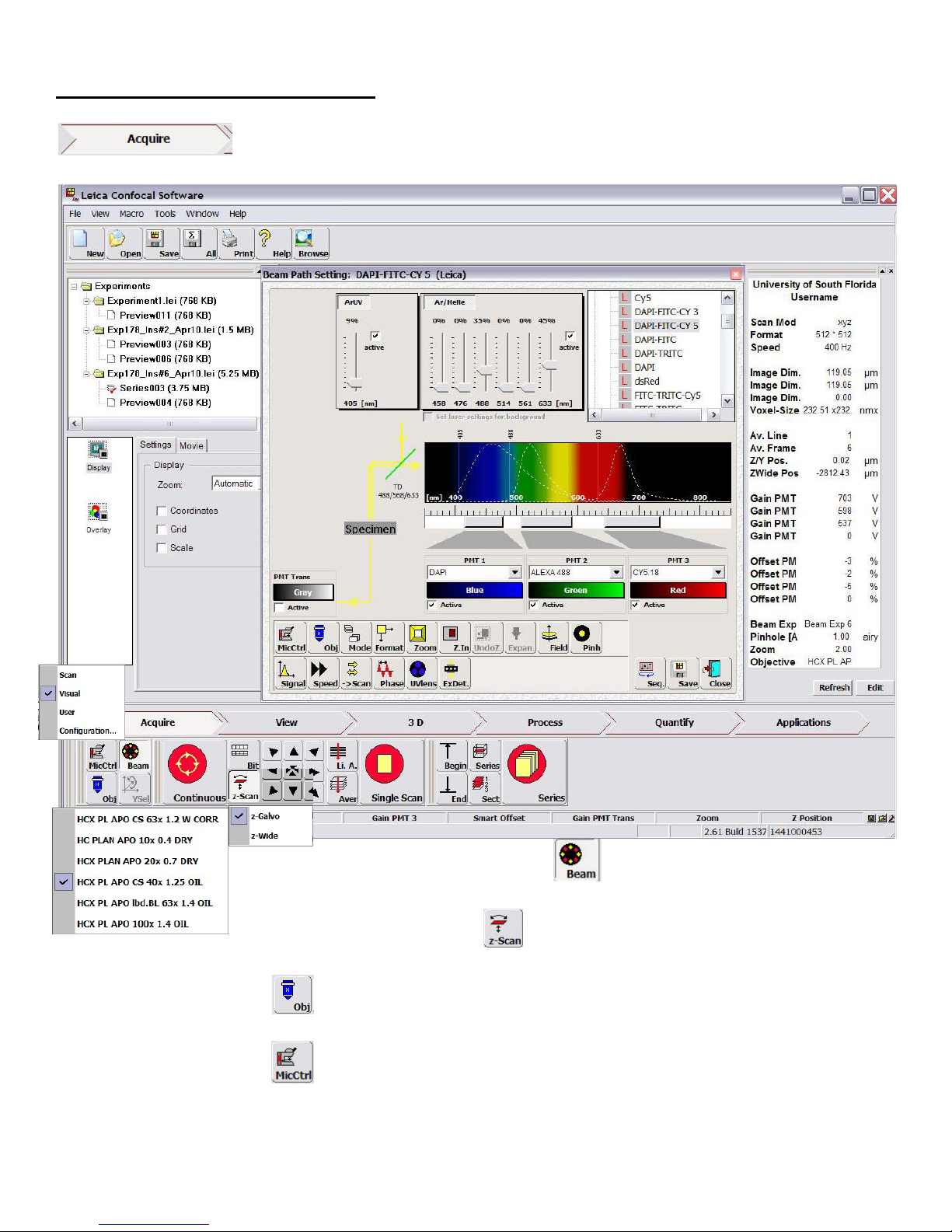
SequentialScanningMode
Thisconfocalmicroscopycandetectupto
fourchannels(3fluorescenceand1
transmission)simultaneouslyaslongasall
theexcitationandemissionspectraof
fluorophoresarewellseparated.Imagingof
samplesstainedwithdifferentdyesthus
simplyrequiresmanipulationoflaserpower
andadjustmentofthedetectorbandwidth.
However,oftenexcitationofone
fluorophoremaycausetheemissionto
appearintotherangeofanother(bleed‐
through)orcanbeinducedbyneighboring
laserlines(cross‐talk),bothofwhich
producefalsesignals.Toavoidthese,the
sampleshouldbescannedsequentiallyby
collectingonefluorophoresignalatany
giventime.Therearetwowaystosetup
sequentialscanmodedependingon
fluorescenceofyoursample.
Toscansequentiallythesamplewithoutneedtouse405nmUVlaser(noDAPIstaining):
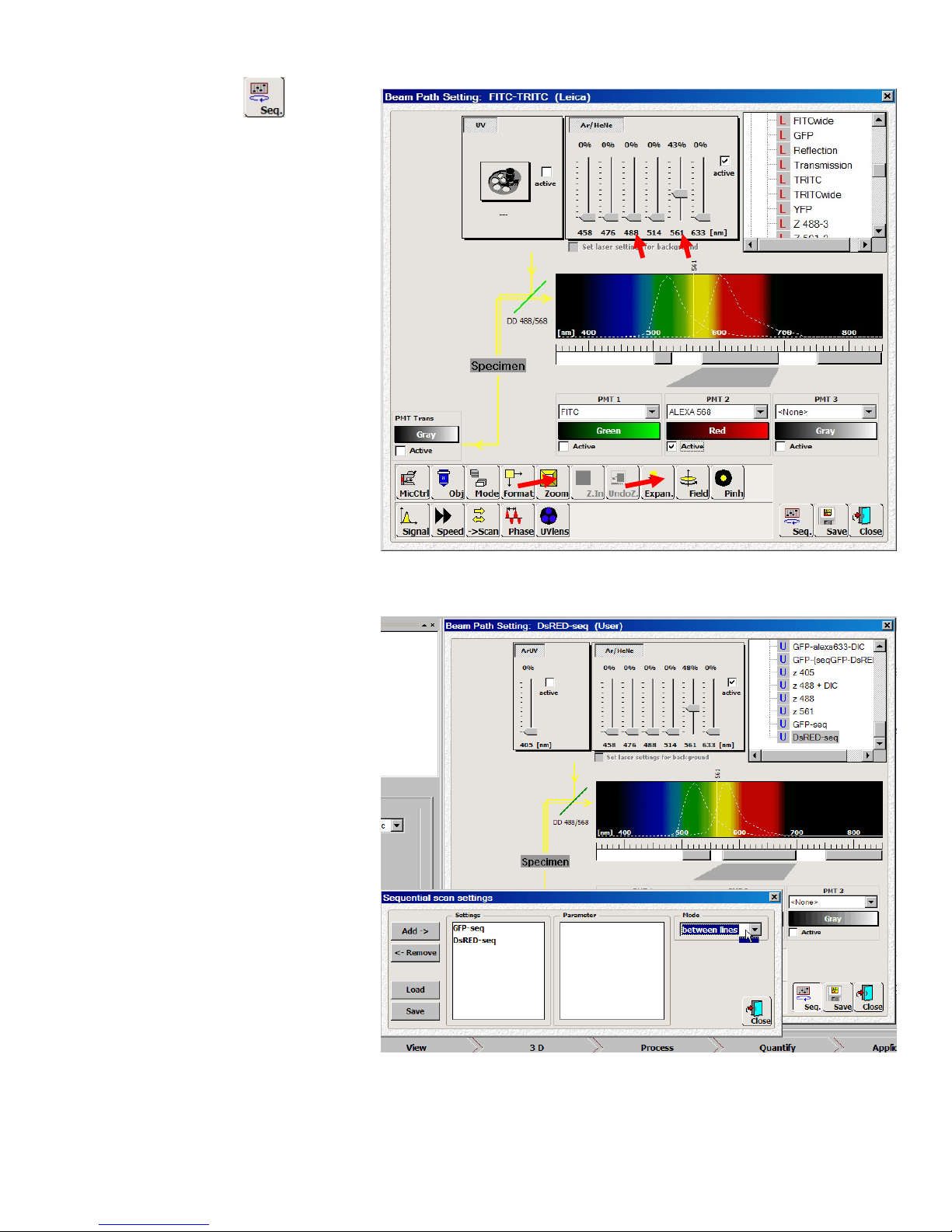
1. Choosethebeamsettingparameter
foryourfluorophores(forexample,
GFPandDsRED)byselectingtheLeica
FITC‐TRITCfromthepresetlist).Itwill
setlaserlines,dichroicmirror,and
PMTsforsimultaneousimagingofthe
twocolors.
2. Setupaconditionforone
fluorophore(i.e.FITC)byonly
activatingandadjustingthelaserlevel
andthePMT(i.e.decreasethe561
nmlaserpowerto0%anduncheck
thePMT2Activebox).
3. ClickSavebuttonbontheBeamPath
Settingpanelandtypeanameforthis
setupasyourownsetting(i.e.“GFP‐
seq”).ClickOKandthesettingwillbe
intheUsersetlist(Uinfrontofthe
settingname).
4. Repeatthesameprocessfortheotherfluorophore(i.e.TRITC)andsavetheconditionasanothersetting
(i.e.“DsRED‐seq”).MakesuretouncheckthePMT1Activecheckboxandlower488laserlevelto0%.
b