ENG
38
NEBULIZER THERAPY – WHAT IS IT?
Nebulizer is a device for formation and spraying of aerosol. The word “nebulizer” is derived from the
latin word “nebula” (fog, cloud) and was first used in 1874 for a device that turns a liquid substance into
an aerosol for medical purposes. One of the first portable “aerosol apparatuses” was created by j. Sales-
girons in paris in 1859. The first nebulizers were used as steam jet energy sources and were applied for
inhalation the vapors of resins and antiseptics by tuberculosis patients. Presently, the term “inhaler” is
often used instead of “nebulizer”.
The purpose of the nebulizer therapy is to quickly deliver to the respiratory passages a therapeutic doze
of a preparation in aerosol form. Continuous supply of aerosol allows, within several minutes, creating
high concentration of a medicine in the upper and lower respiratory passages and lungs, with low prob-
ability of any by-effects. Respectively, effective bronchodilation (bronchi expansion) is reached, and the
need for hospitalization is eliminated or the hospital stay is reduced.
Little Doctor Internatiоnаl (S) Pte. Ltd. offers you to use inhaler LD-210C, whose distinctive features are
the possibility to use a wide range of medicines, low inhalation solution residual volume, and reliable
and simple use. We thank you for your choice.
GENERAL INFORMATION
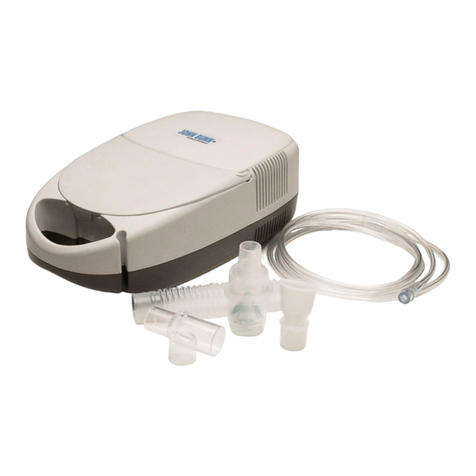
Compressor nebulizer LD is designed for treating the diseases of respiratory passages and lungs by med-
icine solution aerosols.
This Instruction Manual is designed to assist the user with safe and effective operation of the Compressor
Nebulizer LD.
Use this Device according to the rules described in this Manual. Operate the Device only as intended. Do not
use the Device for any other purposes. Read and understand the whole Instruction Manual.
Functionally, the device consists of an air compressor and nebulizer (aerosol formation chamber). The
air compressor, on/off power switch and air filter are united in one casing. From the air compressor, the
compressed air is fed through a pipe to the nebulizer, where aerosol is formed. For cooling the compres-
sor, air is force-feed into the device’s casing.
INDICATIONS FOR USE
The LD compressor nebulizer is designed for the treatment of respiratory diseases such as rhinitis,
pharyngitis, laryngotracheitis, acute and chronic bronchitis, bronchial asthma.
CONTRAINDICATIONS
The LD compressor nebulizer is сontraindicated for use if there are malignant neoplasms, systemic
blood diseases, severe general exhaustion, stage III hypertension, pronounced atherosclerosis of the
cerebral vessels, diseases of the cardiovascular system in the stage of decompensation, nosebleeds or
a predisposition to nosebleeds, hemoptysis, fever (body temperature above 38 °C), active pulmonary
tuberculosis, acute pneumonia, hypertrophy of the mucous membranes of the respiratory tract, tonsillitis,
pleurisy, epilepsy with frequent seizures, hysteria with severe convulsive seizures, psychosis with symptoms
of psychomotor agitation, alcohol or drug intoxication, individual intolerance to procedures, general
severe patient status. If the patient has recently undergone dental surgery or if the patient is undergoing
treatment related to problems in the mouth or throat, it is necessary to consult a doctor before use of the
device.