ENG
19
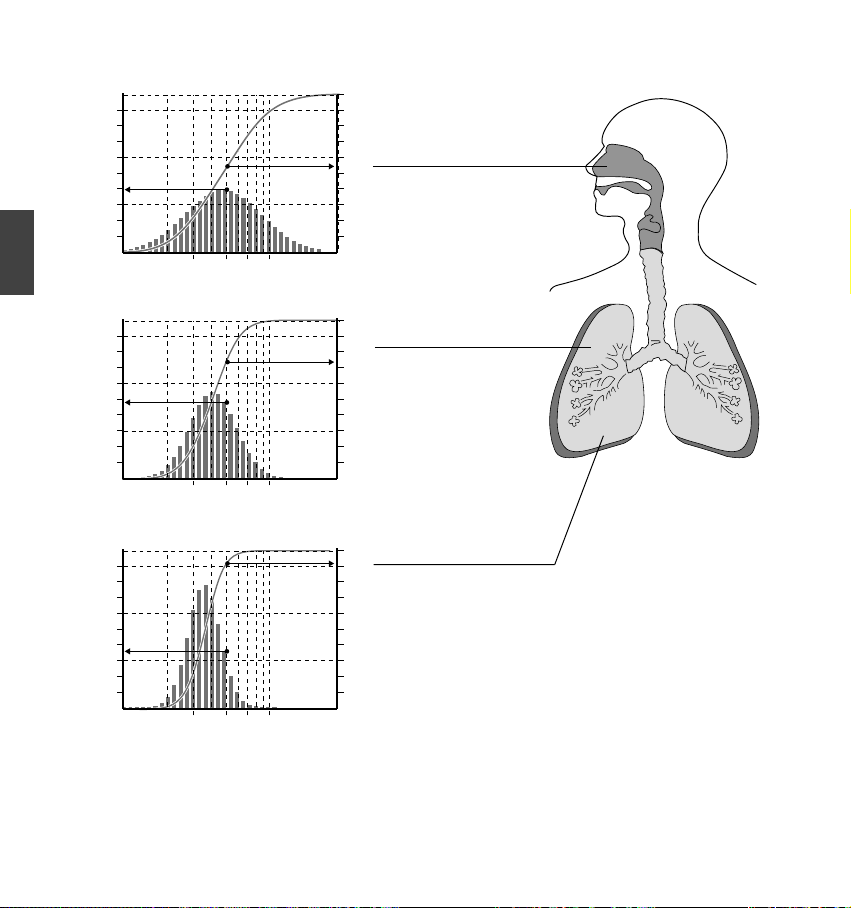
each time you use the device.
●Do not leave the device or its parts where it will be exposed to extreme temperatures or changes in
humidity, such as leaving the device in a vehicle during warm or hot months, or where it will be exposed to
direct sunlight.
CAUTION
●Limit the use of the device to 20 minutes at a time, and wait 40 minutes before using the device again.
●Provide close supervision when this device is used by, on, or near infants, children or compromised
individuals.
●Do not insert any object into the compressor.
●Make sure that the air filter is clean. If the air filter has changed color or has not been used for 60 days,
replace the filter.
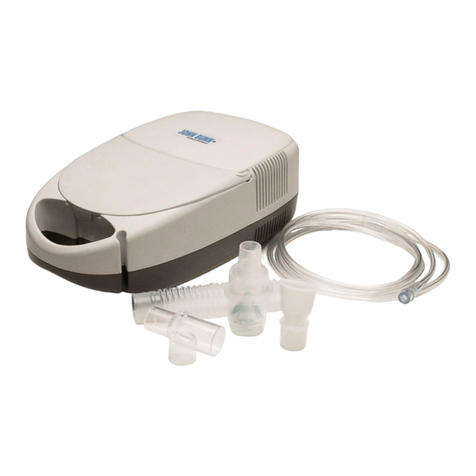
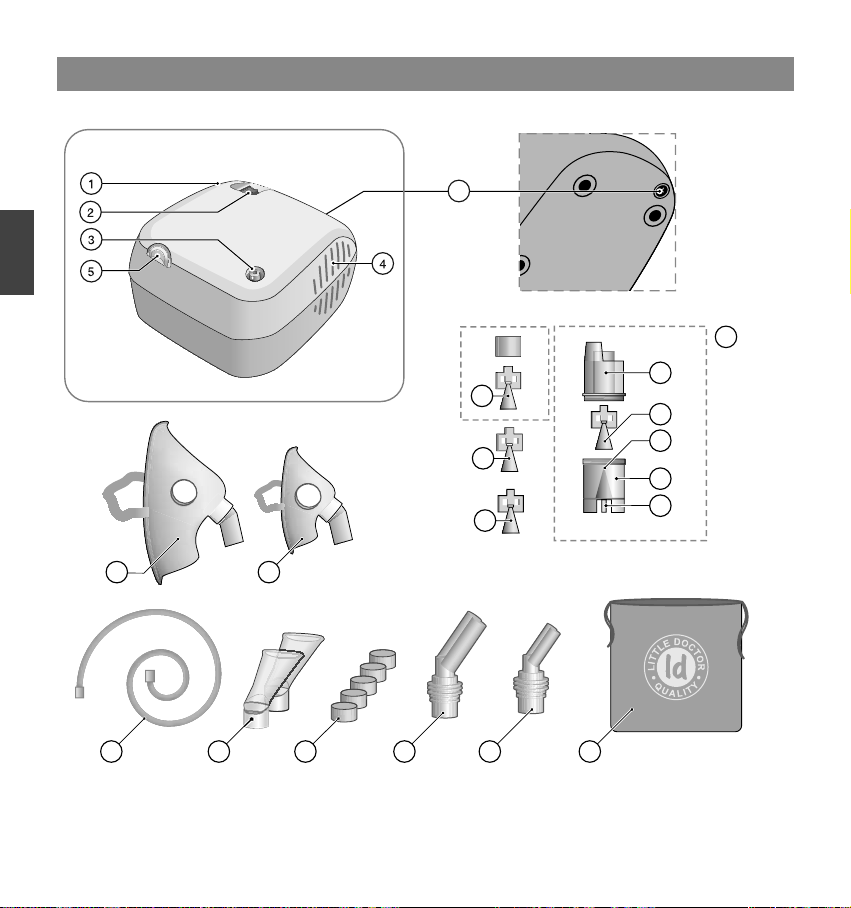
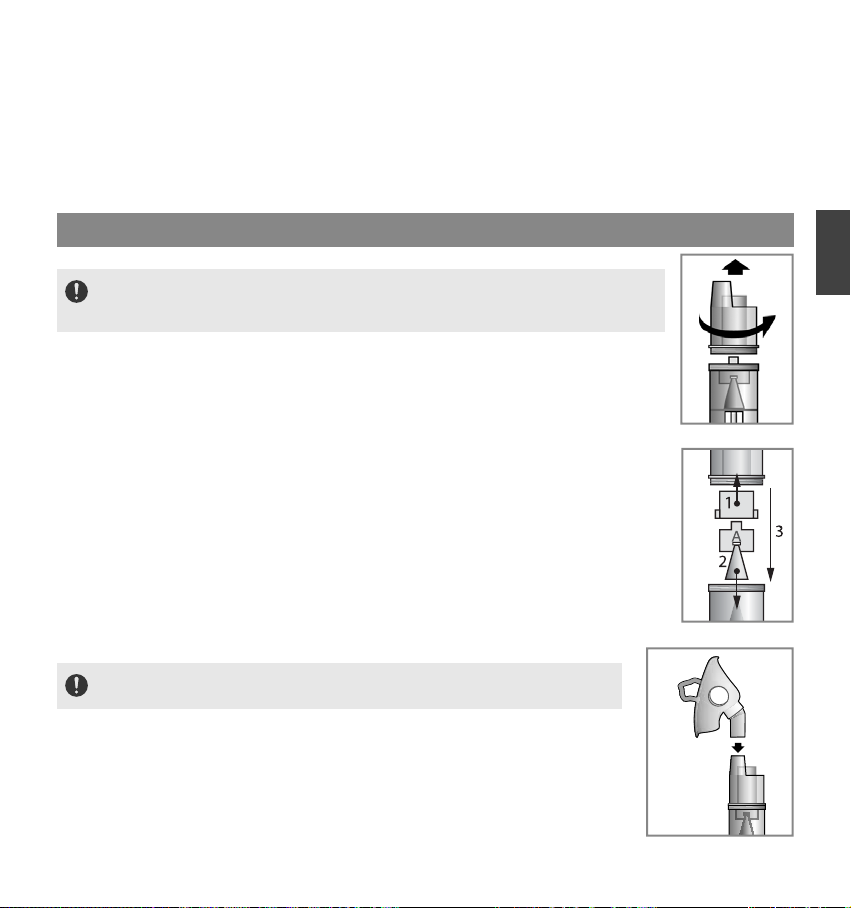
●Make sure that the nebulizer kit is correctly assembled, the air filter is properly installed, and the air tube is
correctly connected to the compressor and the nebulizer kit. Air may leak from the air tube during use if not
securely connected.
●Inspect the compressor (main unit) and the nebulizer parts each time before using the device. Make sure no
parts are damaged, the nozzle and air tube are not blocked and the compressor operates normally.
●Do not use the device if the air tube is bent.
●Do not block the air filter cover.
●Do not alter the baffle, the nozzle in the medication tank or any part of the nebulizer kit.
●Do not add more than 10ml of medication to the medication tank.
●Do not operate the device at temperatures greater than 40˚C.
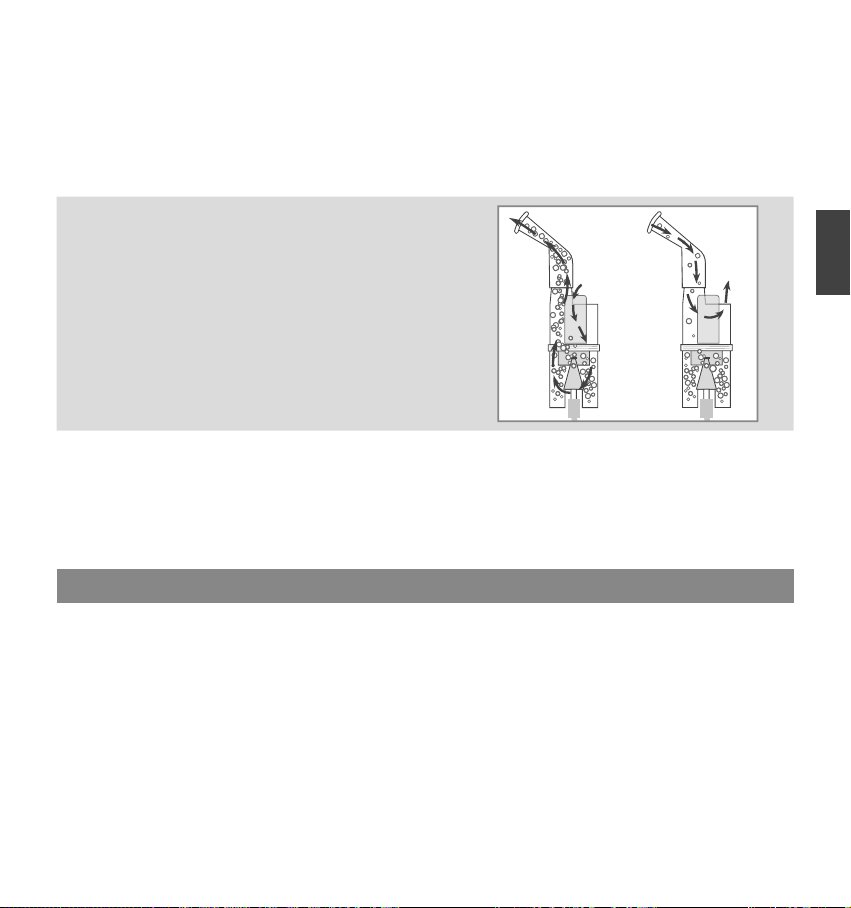
●Do not tilt the nebulizer kit so the angle of the kit is greater than 45˚. Medication may flow into the mouth.
●Do not shake the nebulizer kit while using the device.
●Do not subject the compressor, or any of the components to strong shocks, such as dropping on the floor.
●This device is approved for human use only.
●Do not disassemble or attempt to repair the device or components.
●Use the device only for its intended use as described in the instruction manual. Do not use attachments not
recommended by the manufacturer.
●Dispose of the device, components and optional accessories according to applicable local regulations.
Unlawful disposal may cause environmental pollution.
●Make sure that the air tube is securely attached to the compressor (main unit) and nebulizing parts,
and does not come loose. Twist the air tube slightly when inserting it into the connectors to avoid the tube
disconnecting during use.
RISK OF ELECTRICAL SHOCK
●Do not use the compressor (main unit) and the power cord while they are wet.
●Do not plug or unplug the power cord into the electrical outlet with wet hands.
●Do not immerse the compressor (main unit) in water or other liquid.
●Do not spill water or other liquids on the compressor .These parts are not waterproof. If liquid spills on
these parts, please unplug the power cord and wipe off the liquid with gauze or other soft absorbent material
immediately.
●Do not use or store the device in humid locations or outdoors. Use the device within the operating
temperature and humidity.