ENG
8
Performing the inhalation.
The length of one treatment session should not exceed 20 minutes. Consult your attending physician
about the length of the inhalation procedure.
You should always be calm and relaxed during the inhalation. Breathing should be slow and deep, so
that the preparation could fill the lungs well and reach the deep portions of bronchi.
Briefly hold your breath, and then exhale slowly. Do not attempt to breathe too rapidly. Make pauses if
you feel that you need it.
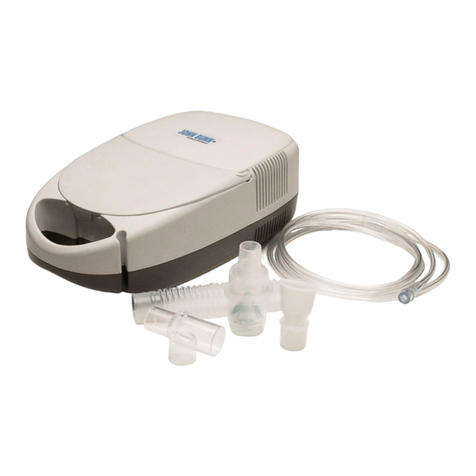
Breath-actuated nebulizer.
The special design of the nebulizer in the form of chambers con-
nected in a certain manner provides different ways of air streams
during inhaling and exhaling.
It allows obtaining the air stream with greatest aerosol concentration
when inhaling and reducing aerosol loss when exhaling. The effec-
tiveness of inhalation using the breath-actuated nebulizer is increased
significantly.
Completing the inhalation.
When the inhalation solution is used up and the inhalation time recommended by the doctor has
expired, turn the device off by putting the tumbler in «O» position and unplug it.
After inhalation, breathe fresh air for some time for better treatment effect.
After each application of the device, the residual preparation should be removed out of it. Clean and wash
the device as described in paragraph 1 «
CARE, STORAGE, REPAIR AND DISPOSAL
».
CARE, STORAGE, REPAIR AND DISPOSAL
1. Regularly clean the device and all accessories. It is recommended that all the accessories should
be wiped with a 3% solution of hydrogen peroxide with addition of 0.5% solution of a detergent (for
example, a laundry powder). After that, the nebulizer should be washed by a rich jet of water. The
mouthpieces and nose nozzles may be treated by boiling for 10 minutes or autoclaving at 150°С. After
the treatment, wipe dry all parts of the device with a soft cloth.
2. Regularly check the filter for dirt and replace it when needed.
FILTER REPLACEMENT IS
RECOMMENDED AT LEAST ONCE A YEAR.
3. Keep this Device from exposure to direct sunlight, shocks.
4. Do not keep and use this Device near heating installations and open fire.
5. Keep the Device clean and protect it from dust.
6. Contact of device with aggressive solutions is not allowed.
7.
Repair the Device only in authorized organizations.
8.
On expiration of the warranted service life apply from time to time to authorized repair organizations to
check the technical condition of the Device. Dispose to time to authorized repair organizations to check the
technical condition of the Device. Dispose of the Device and its components according to the application
local regulations. No special requirements to disposal of this Device are defined by the manufacturer.