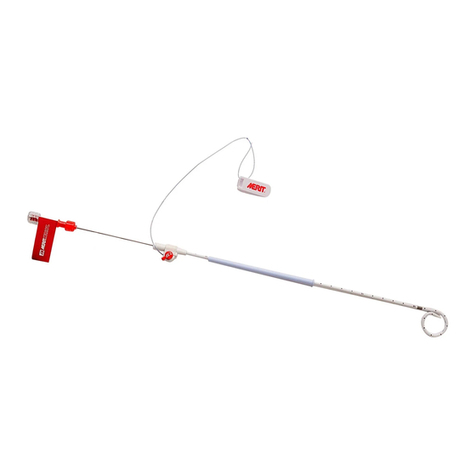
60ml Ination Syringe
INSTRUCTIONS FOR USE
DESCRIPTION:
The BIG60TM Inflation Syringe is a 60ml disposable inflation device
capable of producing a maximum pressure of 12 ATM/BAR, fitted
with a threaded plunger assembly with a lock/release bar, a
flexible high pressure tube, and a three-way medium pressure
stopcock. The BIG60TM Inflation Syringe is designed to generate
and monitor inflation pressures over a range of -.68 to 12ATM/
BAR (-10 to 176 psi) with accuracy of 3% ATM/BAR.
INTENDED USE:
This inflation device is used to inflate and deflate an angioplasty
balloon or other interventional device, and to measure the
pressure within the balloon.
PRECAUTIONS:
• Inspect the BIG60TM Inflation Syringe and packaging
for damage prior to use. Do not use product if opened
or damaged. Confirm the product is consistent with the
package label. Contact Customer Service to report and
replace damaged product.
• For single patient use only. Do not reuse,reprocess,
or resterilize. Reuse, reprocessing, or resterilization
may compromise the structural integrity of the device
and/or lead to device failure which, in turn, may result
in patient injury, illness, or death. Reuse, reprocessing, or
resterilization may also create a risk of contamination
of the product and/or cause patient infection or cross-
infection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another.
Contamination of the product may lead to injury, illness,
or death of the patient.
• Graduations on syringe barrel are for reference only
and are not intended for exact measurement.
INSTRUCTIONS FOR USE:
1. Prior to use, free the plunger tip by twisting the
syringe plunger/handle 360 degrees clockwise.
2. To prep syringe, simply aspirate up to 60ml of contrast
solution into the syringe by pulling back on the syringe handle.
CAUTION: Inspect the syringe tubing and stopcock (if used) to
ensure there is no air in the system.
3. Remove any excess air by orienting the syringe upwards,
squeezing the trigger located on the syringe handle and pushing
the handle forward. Push forward until the syringe plunger tip
is oriented with the black arrow printed on the syringe barrel
(approximately 35ml).
ATTACHING THE INFLATION SYRINGE TO THE BALLOON:
NOTE: Refer to the manufacturer’s directions accompanying
the balloon dilation catheter, or other interventional device,
for specific information on use, maximum inflation pressure,
precautions, and warnings for that device.
1. Prepare and test the balloon catheter according to the
catheter manufacturer’s instructions for use.
2. Create a fluid-fluid connection between the balloon and the
inflation syringe inspection extension tube and connect the luer
connectors securely.
3. Squeeze the trigger and pull back on the handle to
apply vacuum to the balloon.
BALLOON INFLATION AND DEFLATION:
1. To inflate the balloon, squeeze the trigger allowing the
plunger to return to the resting position (0 ATM/BAR). Release
grip on the trigger, locking the plunger into position.
2. To increase pressure, rotate the handle clockwise until the
desired inflation pressure is reached. The locking mechanism
maintains the pressure.
CAUTION: Do not exceed 12 ATM.
NOTE: Loss of pressure may indicate a leak in the system.
3. To deflate the balloon, (rotate the handle counter-clockwise
to relieve pressures above 6 ATM) squeeze the trigger and pull
the handle back to generate the desired negative pressure.
Release the grip on the trigger to lock the plunger in the negative
pressure position.
STORAGE: Store in a cool, dry place.
WARRANTY
The manufacturer warrants that reasonable care has been used in the design and
manufacture of this device. This warranty is exclusive and manufacturer makes
no other representations or warranties of any kind to customers, its end users, or
to any third parties with respect to the device and hereby expressly disclaims any
and all other warranties, express or implied, statutory or otherwise, including, but
not limited to, infringement and the implied warranties of merchantability and
tness for a particular purpose, even if manufacturer is aware of such purpose.
Handling and storage of this device, as well as other factors relating to the
patient, diagnosis, treatment, implant procedures, and other matters beyond the
control of the manufacturer, directly aect the device and the results obtained
from its use. The manufacturer’s obligation under this warranty is limited to the
replacement of the device. Under no circumstances shall manufacturer be liable
to customer or any other person or entity for any punitive, special, incidental or
consequential damages directly or indirectly arising from the use of this device.
The manufacturer neither assumes, nor authorizes any other person to assume for
it, any other or additional liability or responsibility in connection with this device.
This warranty shall not apply, and manufacturer assumes no liability with respect
to, devices that have been (i) modied, changed, altered, misused, mishandled,
repaired, reused, reprocessed, refurbished or resterilized; (ii) subjected to improper
maintenance, testing or storage, accident, tampering, or inadequate protection
against shock, vibration, excessively high or low temperatures, overpressure, or
physical, environmental or electrical stress; (iii) been used outside the approved
“Indications for Use” as cleared by the relevant competent authority, used
contrary to the use outlined in the device specications, or in an application
or environment for which such device was not designed or contemplated; or
(iv) distributed or used contrary to applicable federal, state, local or regulatory
standards.
RX only: CAUTION: Federal (U.S.A.) law restricts this device to
sale by or on the order of a physician.
Single Use
Caution: Consult accompanying document
Sterile if package is unopened or undamaged
Non-pyrogenic
Manufacturer: www.merit.com
Merit Medical Systems, Inc. South Jordan, Utah 84095 U.S.A.
1-801-253-1600
U.S.A. Customer Service 1-800-356-3748
Authorized Representative:
Merit Medical Ireland Ltd, Parkmore Business Park West,
Galway, Ireland
401996001/A ID 111110