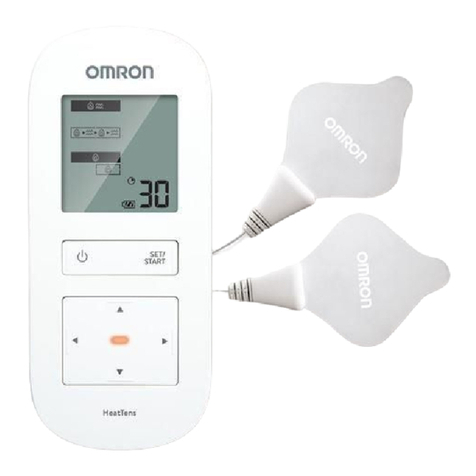
Attach one pad below and above the
region in pain, both on same side.
Attach both pads on the lower back according to your pain.
Place pads on muscle of back, not on spine, for optimal therapy.
LOWER BACK
(HIP & THIGH)
Attach both pads on
either side of the area
with pain. (CALF)
Attach both pads on the calf where you feel pain.
Pads should not be placed simultaneously on
the calves of both legs.
Outside
Inside
(ANKLE)
Attach pads on the left for pain on the outside
of your ankle/foot. Attach the pads on the right
for pain on the inside of your ankle/foot.
Do not put the pads on the bottom of both
feet at the same time.
LEG & FOOT
(KNEE)
Attach both pads above the knee or
above and below the joint with pain.
(ELBOW)
Attach both pads on either side of
the joint with the pain.
JOINT
STEP 2 – SELECT 1 OF 5 MODES
Press the Power button.
Press ▲(Up) or ▼(Down) button to choose 1 of the 5 modes.
Modes cannot be combined.
Press the Set/Start button to select the mode.
It will start the therapy at the intensity level of 1.
Select a pain mode:
1. Arm/Shoulder
2. Lower Back
3. Leg/Foot
Select a massage-like mode:
4. Knead
5. Steady
How to change modes during therapy?
If you want to change modes during therapy, press the Set/Start button and ▲(Up) or ▼(Down) button to select a new mode. You can only
use ONE MODE at a time.
If you don’t press the Set/Start button to select the mode, the unit will automatically turn off after 3 minutes.
How to select the right mode?
Any of the modes can be used on body parts or pains described in this manual or Quick Start Guide/Pad Placement Guide.
Select the mode that feels right for your unique pain.
Therapies
designed for Arm/Shoulder Lower Back Leg/Foot Knead Steady
Mode Light
and the Back of
Main Unit
Potential
conditions
Stiffness, sore or achy,
tight feeling.
Stiffness, soreness,
muscle spasm, nerve
pain.
Swelling, fatigue,
chilly feeling, sore or
achy.
Stiffness, soreness,
tight feeling.
Stiffness, soreness,
tight feeling, or achy.
What does
the therapy
deliver?
Series of low to
high rate tapping,
pulsing, kneading
and massage-like
sensations.
Series of high rate to
low tingling sensations,
followed by tapping.
With higher intensity,
you may feel kneading or
massage-like sensations.
Series of low rate
tapping, pulsing
sensations.
Series of medium rate
pulsing sensations to
mimic massage.
Series of regular
pulsing sensations that
do not change.
STEP 3 – SELECT INTENSITY LEVEL (1 LOW – 10 HIGH)
The unit automatically starts at the intensity level of 1. Slowly increase the intensity level by pressing ▲(Up) button. You should feel a
gentle pulsing sensation.
How do I select the right intensity level for my pain?
Each time you press ▲(Up) or ▼(Down) button, it moves to another level. If the stimulation sensation becomes weaker or disappears,
increase the intensity. But, if the sensation is at all uncomfortable, press ▼(Down) button to decrease the intensity.
• Press ▲ for higher intensity.
• Press ▼ for lower intensity.
If Pad Light is blinking, the unit will automatically turn off after 30 seconds. ( “TROUBLE SHOOTING”)
What intensity level is my unit on?
Press ▲(Up)/▼(Down) button to increase/decrease the intensity. The Intensity Light moves up/down after pressing it once or twice (as shown
below). Therefore, the light may not move up/down, but the intensity level does increase/decrease each time you press it.
ntensity Light:
ntensity level 1 or 2 3 or 4 5 or 6 7 or 8 9 or 10:
How long is the therapy?
The unit will continue automatically for 15 minutes before it shuts off. If you want to stop the therapy while in use, press the Power button.
We recommend a total of 30 minutes therapy in one sitting, up to 3 times/day.
HOW TO CONTROL AND REDUCE YOUR PAIN
When should you start therapy?
Use as soon as your pain begins. Start with one session (the unit automatically turns off at 15 minutes). Turn off with pads still on and RATE
YOUR PAIN again (1 low to 10 high).
Get to your pain early
If you get to your pain early, it may prevent the pain from becoming worse, or even chronic. It’s better for you to get it under
control sooner so that it does not reach a high pain threshold where it limits your daily activities.
How long should you use it?
Start with one 15 minute session. Always turn unit off with pads still on. Rate your pain to check your progress, 1 low to 10 high. Stop therapy
session if pain has reduced or stopped. Press the Power button to continue therapy for another 15 minute session.
1 session: 15 minute automatic shut-off Max minutes/session: 30 minutes Max times/day: 3 times
See warnings. Long-time treatment and strong stimulation may cause muscular fatigue and may generate adverse effects.
When to stop using the unit?
1. If you experienced an adverse reaction (skin irritation/redness/burns, headache or other painful sensation, or if you feel any unusual
discomfort).
2. If your pain does not improve, becomes seriously chronic and severe, or continues for more than fi ve days.
What type of pain is it best for?
This therapy works best on acute pain because it is localized. Acute pain is pain in one area for less than 3 months. If you have chronic pain,
you may have pain in more than one area and for longer than 6 months. Chronic pain may be compounded by other issues that this unit
cannot address.
Remember, this unit does not cure your pain or the original cause of the pain. It provides temporary relief or reduction of pain so that you can
control your life and activities better.
Before using, check these points to make sure everything is working properly.
1. Make sure the cord is not broken.
2. Check that the pad adhesive sticks and is not damaged.
3. The electrode cord connection is not broken.
4. The unit is intact and in working order.
5. There is no battery leakage.
CLEANING AND STORAGE
The unit is designed for repeated use over time. The pads will last up to 150 uses, or 5 months (based on use 1/day). Here are
important cleaning and storage instructions:
Cleaning the pads
1. Turn the power off and remove the electrode cord from the pads.
2. Wash the pads when the adhesive surface becomes dirty and/or the pads are diffi cult to attach.
• Wash the pad softly with your fi ngertips under slow running cold water for several seconds (do not use a sponge/
cloth/sharp object like a nail on adhesive side, do not use detergents, chemicals or soap).
3. Pads can be washed after 15 uses, approximately ten times for up to 150 uses. Do not wash the pads too long or too frequently.
4. Dry the pads and let the adhesive surface air-dry completely. Do not wipe with a tissue paper or cloth.
5. Pads are replaceable and can be purchased when needed by calling 1-800-634-4350 or go to omronhealthcare.com.
The life of the pads may vary by how often you wash the pads, the skin condition, and how you store the pads.
When should you replace your pads?
If the pad no longer sticks to your skin or if more than 25% of the pad’s surface is not in contact with your skin.
Cleaning the unit
1. Turn unit off and disconnect the electrode cords from the pads.
2. Clean with a lightly moistened cloth (or a cloth soaked in a neutral cleaning solution) and wipe gently.
• Do not use chemicals (like thinner, benzene).
• Do not let water get into the internal area.
Storing the pads
1. Turn the unit off and remove the cord from the bottom of the unit.
2. Remove the pads from your body.
3. Leave the electrode cords connected to the pads.
Place the pads on the pad holder, one pad on each side with the sticky side of each pad on the pad holder.
4. Wrap the electrode cords around the pad holder.
Storing the unit and pads
• Place the unit, pads with electrode cords on pad holder, Pad Placement Guide and Instruction Manual inside the original box.
• Do not keep in areas subject to direct sunlight, high or low temperatures, humid area, near to fi re, vibration, or shock.
Storage temperature, 32°F - 104°F (0°C - 40°C), 30% - 80% relative humidity.
• Do not keep at places that can be easily reached by children.
• When not in use for a long period, remove the batteries before storage, to avoid liquid discharge from batteries.
• Do not wrap the electrode cords around the unit because it may damage the cord.
TROUBLESHOOTING
If this happens... Possible causes... Try this solution...
The intensity is not felt.
Very weak intensity
level.
Are you using only 1 pad? Put the other pad on your skin. You must use BOTH PADS
for therapy to work.
Have you removed the transparent fi lm from the
pad?
Peel off fi lm on the adhesive surface of pads.
Are the pads stacked together or do pads
overlap?
Check placement of pads. Refer to Pad Placement Guide.
Is the cord properly connected to the unit? Connect cord plug correctly into the jack at bottom of this
unit.
Is the intensity setting getting weak? Press the ▲(Up) button.
Is the gel damaged? Replace pad.
Are the batteries weak? Replace both AAA batteries.
Is the intensity “1”? Press the ▲(Up) button.
The skin turns red or the
skin feels irritated.
Is the adhesive surface of pads dirty or dry?
Wash adhesive surface of pads softly with your fi ngertips for about 3
seconds under slow running water.
Is therapy time too long? Use less than 15 minutes.
Are the two pads attached properly to the
body?
Refer to the Pad Placement Guide and attach correctly.
Is the pad surface worn out? Replace both pads at the same time.
No power source. Are the polarities of battery (+ and -) aligned
in the wrong direction? or Are the batteries
depleted?
Check batteries for correct alignment. or Replace batteries.
Power cut off during use. Are the batteries weak? Replace both batteries at the same time.
Is the cord broken? Replace cord.
Battery Light lights up.
Are the batteries weak? Replace both batteries at the same time.
Pad gel does not stick to
skin.
Have you removed the transparent fi lm from the pad?
Peel off fi lm on the adhesive surface of pads.
Is the pad wet? or Is your skin too wet? Dry the pad. or Dry the skin.
The pad gel may be damaged. Replace the pad.
Is there too much hair on your skin?
Shave the immediate area for proper pad adhesion.
Is the adhesive surface of pads dirty or dry? Wash adhesive surface of pads softly with your fi ngertips for
about 3 seconds under slow running cold water.
Are you using pad during perspiring? Dry the pad placement area.
Have the pads been washed too long and/ or
too frequently?
Leave the pad in freezer for overnight.
Were the pads stored under high temperature,
high humidity, or direct sunshine?
Replace both pads.
Pad Light is blinking. Are both pads attached to the body? Re-attach dislocated pad(s) onto the skin fi rmly.
Have you removed the transparent fi lm from the pad?
Peel off fi lm on the adhesive surface of pads.
Is the cord properly connected to the main
unit?
Connect cord plug correctly into the jack at the bottom of the
main unit.
Is the adhesive surface of pads dirty or dry? Wash adhesive surface of pads softly with your fi ngertips for
about 3 seconds under slow running cold water.
If the above measures are not effective, contact us at 1-800-634-4350.
LIMITED WARRANTY
Your OMRON®Pocket Pain Pro™ unit, excluding the batteries, is warranted to be free from defects in materials and workmanship appearing
within 1 year from the date of purchase, when used in accordance with the instructions provided. The pads supplied with the unit are
warranted for 30 days. The above warranties extend only to the original retail purchaser. We will, at our option, replace without charge, any
unit covered by the above warranty. Replacement is our only responsibility and your only remedy under the above warranties.
To obtain warranty service, contact Customer Service by calling 1-800-634-4350 for the address of the Inspection Center and
shipping and handling charges that may apply. Enclose the Proof of Purchase. Include a letter, with your name, address, phone number,
and description of the specifi c problem. Pack the product carefully to prevent damage in transit. Because of possible loss in transit, we
recommend insuring the product with return receipt requested.
THIS WARRANTY GIVES YOU SPECIFIC LEGAL RIGHTS, AND YOU MAY HAVE OTHER RIGHTS THAT VARY FROM STATE
TO STATE (OR BY COUNTRY OR PROVINCE). THE FOREGOING IS THE SOLE WARRANTY PROVIDED BY OMRON IN
CONNECTION WITH THIS PRODUCT, AND OMRON HEREBY DISCLAIMS ANY OTHER WARRANTIES, EXPRESS OR IMPLIED,
INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. IMPLIED
WARRANTIES AND OTHER TERMS THAT MAY BE IMPOSED BY LAW, IF ANY, ARE LIMITED IN DURATION TO THE PERIOD
OF THE ABOVE EXPRESS WARRANTY.
SOME STATES (COUNTRIES AND PROVINCES) DO NOT ALLOW LIMITATIONS ON HOW LONG AN IMPLIED WARRANTY
LASTS, SO THE ABOVE LIMITATION MAY NOT APPLY TO YOU. OMRON SHALL NOT BE LIABLE FOR LOSS OF USE OR
ANY OTHER SPECIAL, INCIDENTAL, CONSEQUENTIAL OR INDIRECT COSTS, EXPENSES OR DAMAGES. SOME STATES
(COUNTRIES AND PROVINCES) DO NOT ALLOW THE EXCLUSION OR LIMITATION OF INCIDENTAL OR CONSEQUENTIAL
DAMAGES, SO THE ABOVE EXCLUSION OR LIMITATION MAY NOT APPLY TO YOU.
This warranty provides you with specifi c legal rights, and you may have other rights that vary by jurisdiction. Because of special local
requirements, some of the above limitations and exclusions may not apply to you.
FOR CUSTOMER SERVICE
Visit our web site at: omronhealthcare.com
Call toll free: 1-800-634-4350
SPECIFICATIONS
Product Name OMRON®Pocket Pain Pro™
Model # PM3029
Power Source DC3V (two AAA alkaline batteries or two AAA manganese batteries)
Battery Life
New batteries (two AAA alkaline batteries) will last for approx. 3 months (when used for
15 minutes a day, Lower Back Mode, max. intensity).
Frequency Approx. 1 to 108Hz
PULSE Duration 100 μsec
Maximum Output Voltage 32V (during 500Ω load)
Power Control 10 intensity levels
Operating Temperature, Humidity (When using product) 50°F to 104°F (10 °C to 40 °C), 30 to 80% RH
Storage Temperature, Humidity 32°F to 104°F (0 °C to 40 °C), 30 to 80% RH
Transportation Temperature, Humidity, Air Pressure -4°F to 140°F (-20 °C to 60 °C), 10 to 95% RH, 700 to 1060 hPa
Weight Approx. 75g (incl. batteries)
Outer Dimension Approx. Width 75mm x Height 70mm x Depth 22mm
Classifi cation of ME equipment Internally powered
IP classifi cation IP 22*
NOTE: • These specifi cations are subject to change without notice.
• This OMRON product is produced under the strict quality system of OMRON HEALTHCARE Co. Ltd., Japan.
Designed for a minimum of 5 years life expectancy.
= This shows the Type BF applied part. * Protection against ingress of an object of φ12.5 mm or more. Protection against
the ingress of vertically falling water drips with the device tilted at 15 degrees.
Accessories/replacement parts (To order : omronhealthcare.com)
• PMLLPAD (2.5”x4”) • Plastic Pad Holder-Standard • Electrode Cords
FCC STATEMENT
FCC CAUTION
Changes or modifi cations not expressly approved by the party responsible for compliance could void the user’s authority to operate the equipment.
Note:
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits
are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses and can radiate
radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or
more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any interference received, including interference that may cause undesired operation of this device.
IMPORTANT INFORMATION REGARDING ELECTRO MAGNETIC COMPATIBILITY (EMC)
With the increased number of electronic devices such as PC’s and mobile (cellular) telephones, medical devices in use may be susceptible to
electromagnetic interference from other devices. Electromagnetic interference may result in incorrect operation of the medical device and create a
potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the aim to prevent unsafe product situations, the IEC60601-
1-2 standard has been implemented. This standard defi nes the levels of immunity to electromagnetic interferences as well as maximum levels of
electromagnetic emissions for medical devices.
Medical devices manufactured for OMRON Healthcare conform to this IEC60601-1-2:2007 standard for both immunity and emissions.
Nevertheless, special precautions need to be observed:
• The use of accessories and cables other than those specifi ed by OMRON, with the exception of cables sold by OMRON as replacement parts for
internal components, may result in increased emission or decreased immunity of the device.
• The medical devices should not be used adjacent to or stacked with other equipment. In case adjacent or stacked use is necessary, the medical device
should be observed to verify normal operation in the confi guration in which it will be used.
• Do not use mobile (cellular) telephones and other devices, which generate strong electrical or electromagnetic fi elds, near the medical device. This may
result in incorrect operation of the unit and create a potentially unsafe situation. Recommendation is to keep a minimum distance of 7 m. Verify correct
operation of the device in case the distance is shorter.
The PM3029 is intended for use in the electromagnetic environment specifi ed below. The customer or the user of the PM3029 should assure that it is
used in such environment.
Electromagnetic emissions IEC60601-1-2
Emissions test Compliance Electromagnetic environment - guidance
RF emissions CISPR 11 Group 1 The PM3029 uses RF energy only for its internal function. Therefore, its RF emissions
are very low and are not likely to cause any interference in nearby electronic
equipment.
RF emissions CISPR 11 Class B The PM3029 is suitable for use in all establishments, including domestic
establishments and those directly connected to the public low-voltage power supply
network that supplies buildings used for domestic purposes
Harmonic emissions
IEC 61000-3-2
not applicable
Voltage fl uctuations/ fl icker
emissions IEC 61000-3-3
not applicable
Electromagnetic immunity IEC60601-1-2
Immunity test IEC 60601 Test level Compliance level Electromagnetic environment –guidance
Electrostatic discharge
(ESD) IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floor should be wood, concrete, or ceramic tile. If fl oors are
covered with synthetic material, the relative humidity should
be at least 30 %.
Electrical fast transient/
burst IEC 61000-4-4
±2 kV for power
supply lines
±1 kV for input/
output lines
Not applicable Not applicable
Surge IEC 61000-4-5 ±1 kV line(s) to line(s)
±2 kV line(s) to earth
Not applicable Not applicable
Voltage dips, short
interruptions and voltage
variations on power
supply input lines
IEC 61000-4-11
<5 % UT(>95 % dip inUT)
for 0,5 cycle
40 % UT(60 % dip in UT)
for 5 cycles
70 % UT(30 % dip in UT)
for 25 cycles
<5 % UT(>95 % dip in UT)
for 5 s
Not applicable Not applicable
Power frequency (50/
60 Hz) magnetic fi eld
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fi elds should be at levels
characteristic of a typical location in a typical commercial or
hospital environment.
Note: UTis the A.C. mains voltage prior to application of the test level.
Electromagnetic immunity IEC60601-1-2
Immunity test
IEC 60601 Test level
Compliance level Electromagnetic environment –guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 V rms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 V rms
3 V/m
Portable and mobile RF communications equipment should be used no
closer to any part of the PM3029 including cables, than the recommended
separation distance calculated from the equation appropriate to the
frequency of the transmitter.
Recommend separation distance
d= 1.2 150 kHz to 80 MHz
d= 1.2 80 MHz to 800 MHz
d= 2.3 800 MHz to 2.5 GHz
where Pis the maximum output power rating of the transmitter in watts
(W) according to the transmitter manufacturer and d is the recommended
separation distance in meters (m).
Field strengths from fi xed RF transmitters as determined by an
electromagnetic site survey,*2) should be less than the compliance level in
each frequency range.*3)
Interference may occur in the vicinity of
equipment marked with the following symbol:
Note1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and refl ection from
structures, objects, and people.
*2) Field strengths from fi xed transmitters, such as base stations for radio (cellular/ cordless) telephones and land mobile radio, AM and
FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to fi xed RF transmitters, an electromagnetic site survey should be considered. If the measured fi eld strength in the location in which
the PM3029 is used exceeds the applicable RF compliance level above, the PM3029 should be observed to verify normal operation. If
abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the PM3029.
*3) Over the frequency range 150 kHz to 80 MHz, fi eld strengths should be less than 3 V/m.
Recommended separation distance between portable and mobile RF communications equipment and the PM3029
The PM3029 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customers or
the users of the PM3029 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile
RF communications equipment (transmitters) and the PM3029 as recommended below, according to the maximum output power of the
communications equipment.
Output Power of
Transmitter in Watt
Separation distance according to frequency of transmitter in meter
150 kHz to 80 MHz
d = 1.2
80 MHz to 800 MHz
d = 1.2
800 MHz to 2.5GHz
d = 2.3
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated
using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer.
Note1: At 80MHz and 800MHz, the separation distance for the higher frequency range applies.
Note2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and refl ection from
structures, objects, and people.
Manufactured for : OMRON HEALTHCARE Co., Ltd.
53, Kunotsubo, Terado-cho, Muko, Kyoto, 617-0002 JAPAN
Distributed by: OMRON HEALTHCARE, INC.
1925 West Field Court, Lake Forest, IL 60045 U.S.A.
For questions: 1-800-634-4350 Get general pain info: OmronHealthcare.com
© 2017 OMRON HEALTHCARE, INC. Made in China
Mode
Press
Set/Start Button
Press
Power Button