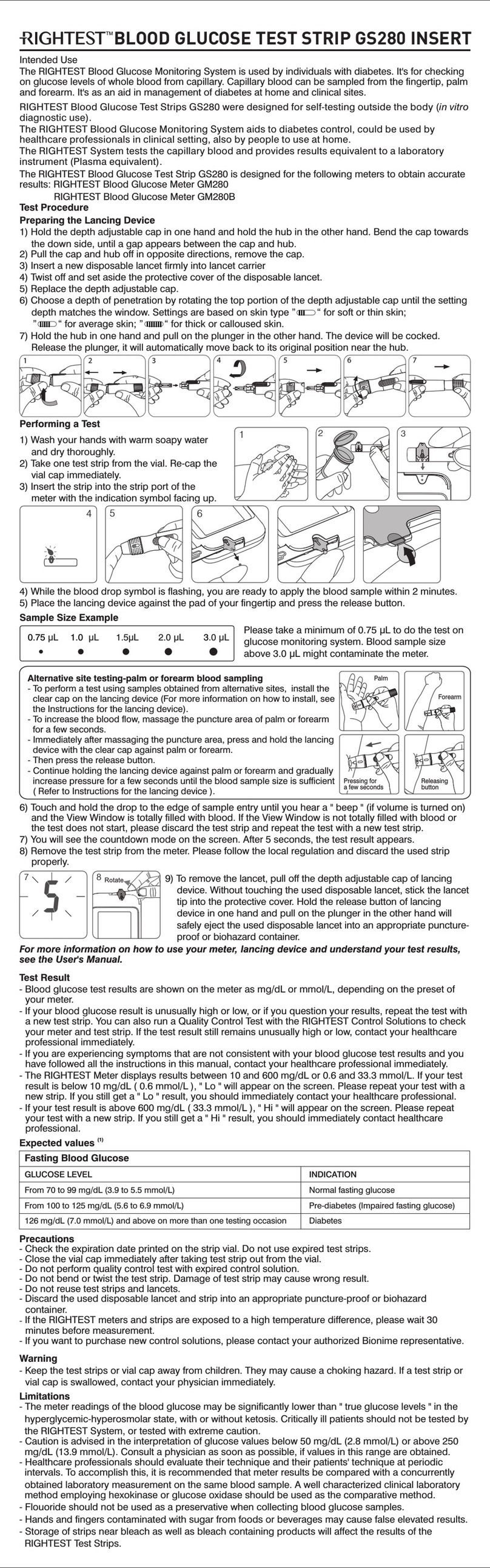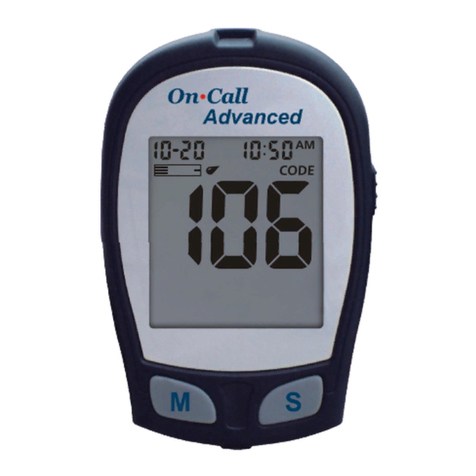
101-3GS570-030
EN
Troubleshooting and Customer Service
For more information on error messages and trouble shooting, please refer to the Error Messages and
Trouble Shooting section of RIGHTEST User's Manual.
If you have any questions or in case of problems with RIGHTEST products, please contact local Bionime
Lay User Evaluation
A total of 110 layperson were enrolled. Each patient tested their fingertip blood samples with 3 lots of Wiz
blood glucose test strip and Wiz Plus meter. Then the professional collected blood samples were
centrifuged immediately after collection to obtain plasma. Analyze the plasma by the lab instrument (YSI
2300 analyzer). 100% of the RIGHTEST Wiz Plus BGMS were within ± 15% of YSI values at glucose
concentrations ≧ 100 mg/dL (5.55 mmol/L) and within ± 15 mg/dL (0.83 mmol/L) at glucose
concentrations < 100 mg/dL (5.55 mmol/L).
Hematocrit
Hematocrit should be between 20 to 60%. If you do not know your hematocrit, ask your healthcare
professional.
Interferences
26 toxic amount tested substances (Acetaminophen, Ascorbic acid, Dopamine, EDTA, Gentisic acid,
Heparin, Ibuprofen, L-dopa, Methyldopa, Pralidoxime iodide, Salicylic Acid, Tetracycline, Tolazamide,
Tolbutamide, Bilirubin, Cholesterol, Creatinine, Glutathione, Haemoglobin, Triglycerides, Uric acid, Maltose,
Xylose, Galactose, Lactose, Icodextrin) in two blood sample concentrations.
Substance and possible level may interfere the glucose measurement:
Ascorbic acid ≧ 6 mg/dL (0.34 mmol/L)
Glutathione reduced ≧ 70 mg/dL (2.28 mmol/L)
Uric Acid ≧ 16 mg/dL (0.95 mmol/L)
Reagents
Each Blood Glucose Test Strip contains the following reagents:
Glucose Oxidase (GOD) 18.8 %
Potassium Ferricyanide 37.7 %
Non-reactive Ingredients 43.5 %
References
1) Diabetes Information - American Association for Clinical Chemistry (AACC)〔Electronic Version〕
Retrieved May. 08, 2019 from www.labtestsonline.org/understanding/analytes/glucose/test.html
2) Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird's Eye View of Glucose Sensing
Enzymes. J Diabetes Sci Technol 2011 Sep; 5(5): 1068–1076 (2011).
Accuracy
The accuracy of Blood Glucose Monitoring System was tested by comparing RIGHTEST Wiz Plus
fingertip whole blood (plasma equivalent) glucose values measured by Blood RIGHTEST Wiz Plus
Glucose Meter with plasma glucose values obtained from a YSI 2300 reference instrument.
The YSI 2300 was calibrated with NIST (SRM) 917c reference.
The results and variations between the two methods, Blood Glucose Monitoring RIGHTEST Wiz Plus
System and YSI 2300 (as the reference method) are shown in the tables below.
Additional Information for Healthcare Professionals
(ii) Control solution:
P-01
300
38.6 (2.1)
1.1 (0.06)
2.8 %
P-02
300
84.7 (4.7)
1.7 (0.09)
2.0 %
P-03
300
136.5 (7.6)
2.5 (0.14)
1.8 %
(i) Venous whole blood sample:
Glucose levels
(1) Total test numbers (n)
(2) Mean mg/dL (mmol/L)
(3) SD mg/dL (mmol/L)
(4) CV (%)
Glucose levels
(1) Total test numbers (n)
(2) Mean mg/dL (mmol/L)
(3) SD mg/dL (mmol/L)
(4) CV (%)
(2)
Detection Principle
The glucose oxidase and potassium ferricyanide in the strip react with the glucose in the sample to
produce an electrical current which is proportional to the amount of glucose in the sample. The meter
measures the current and converts it to the corresponding glucose concentration.
Performance Characteristics
Data generated using RIGHTEST Wiz Plus Blood Glucose Meter. RIGHTEST Wiz Plus Blood Glucose
Meter is the representative of the RIGHTEST Wiz Blood Glucose Meter family (included Wiz and Wiz
Plus Blood Glucose Meter).
Precision
The precision was evaluated including (i) 5 glucose levels of venous whole blood samples (blood
samples were collected with heparin tube and altered for test after 8 hours) (ii) 3 glucose levels of
control solution, in period of 10 days, by 10 meters and 3 batches of strips.
P-04
300
221.2 (12.3)
2.9 (0.16)
1.3 %
P-05
300
329.9 (18.3)
4.4 (0.24)
1.3 %
CS-L
300
37.9 (2.1)
1.0 (0.06)
2.6 %
CS-N
300
85.4 (4.7)
1.5 (0.08)
1.8 %
CS-H
300
246.4 (13.7)
2.6 (0.14)
1.0 %
Quality Control Section
Please refer to the Quality Control section of the User's Manual.
Storage and Handling
- Store the strips in the original capped vial at temperatures between 4°C to 30°C (39°F to 86°F) and relative
humidity below 90 %. Do not freeze.
- Close the vial cap immediately and tightly after taking test strip out from the vial. Do not leave the cap of
vial opened. If the strip is exposed to the air too long, it will absorb the moisture and cause wrong test
result.
- When you open a new vial of test strips please write the first opening date on the label. Use test strips
within 12 months after first opened or until the expiration date printed on the label (whichever comes first).
Measurement Range
The measurement range of RIGHTEST System is 10 to 600 mg/dL or 0.6 to 33.3 mmol/L.
NOTE
- Suggest not to use this meter close to source of strong electromagnetic radiation, to avoid
interference with proper operation.
- Suggest to keep meter free of dust, water or any liquid.
- Flouoride should not be used as a preservative when collecting blood glucose samples.
- Hands and fingers contaminated with sugar from foods or beverages may cause false elevated results.
- The results of blood glucose measurements are different for measurements with whole blood and
plasma.
- Storage of strips near bleach as well as bleach containing products will affect the results of the
RIGHTEST Test Strips.
- RIGHTEST Blood Glucose Test Strips are designed for use with capillary whole blood samples.
Do not use serum or plasma samples.
- Incorrect test results may be obtained at high altitude more than about 3,048 meters (10,000 feet)
above sea level.
- Severe dehydration and excessive water loss may cause inaccurately low results.
- Patients going through oxygen therapy may yield falsely low results.
- RIGHTEST Blood Glucose Monitoring System has not been validated for use on neonates.
Therefore, it should not be used for neonates.
- Do not perform the blood glucose test at temperatures below 10°C (50°F) or above 40°C (104°F), below
10% or above 90% relative humidity. The suggested temperature range for the control solution test is
15 to 40°C (59 to 104°F).
Difference range in values
between the YSI value and
RIGHTEST Wiz Plus meter
The percent ( and number ) of samples was the difference between RIGHTEST
Wiz Plus blood glucose meter and the YSI value within the following intervals.
Difference range in values
between the YSI value and
RIGHTEST Wiz Plus meter
Within ± 5 %
Within ± 10 %
Within ± 15 %
The percent ( and number ) of samples was the difference between RIGHTEST
Wiz Plus blood glucose meter and the YSI value within the following intervals.
*Acceptance criteria in ISO15197 : 2013 are that 95 % of all differences in glucose values should be within
± 15 mg/dL (0.83 mmol/L) at glucose concentrations < 100 mg/dL (5.55 mmol/L), and within ± 15 % at
glucose concentrations ≧ 100 mg/dL (5.55 mmol/L).
Note: For glucose concentrations < 100 mg/dL (5.55 mmol/L), difference values are expressed in mg/dL
(mmol/L), and for glucose concentrations ≧ 100 mg/dL (5.55 mmol/L), difference values are compared in
percentage.
Fingertip
65.0% (277/426)
98.4% (419/426)
100%(426/426)
Palm
62.4%(266/426)
95.5%(407/426)
100%(426/426)
Forearm
59.6%(254/426)
95.1%(405/426)
100%(426/426)
Fingertip
68.2%(135/198)
97.5%(193/198)
100% (198/198)
Palm
68.2%(135/198)
98.5%(195/198)
100% (198/198)
Forearm
53.5%(106/198)
92.4%(183/198)
100% (198/198)
Within ± 5 mg/dL (0.28 mmol/L)
Within ± 10 mg/dL (0.56 mmol/L)
Within ± 15 mg/dL (0.83 mmol/L)
Table 2: Represents samples for glucose results ≧ 100 mg/dL (5.55 mmol/L).
Table 1: Represents samples for glucose results < 100 mg/dL (5.55 mmol/L).
Rev. Date:2019-05
For in vitro diagnostic use Manufacturer
IVD For single use only EC Representive
LOT
Lot number
CE-mark (with No. of notified body)
Consult the instruction for use
Store between tempe rature 4°C and 30°C ( 39°F and 86 °F) Biological risks Expiry date
BIONIME CORPORATION
No. 100, Sec. 2, Daqing St., South Dist.,
Taichung City 40242, Taiwan
Tel: +886 4 2369 2388 Fax:+886 4 2261 7586
Bionime GmbH
Tramstrasse 16
9442 Berneck
Switzerland