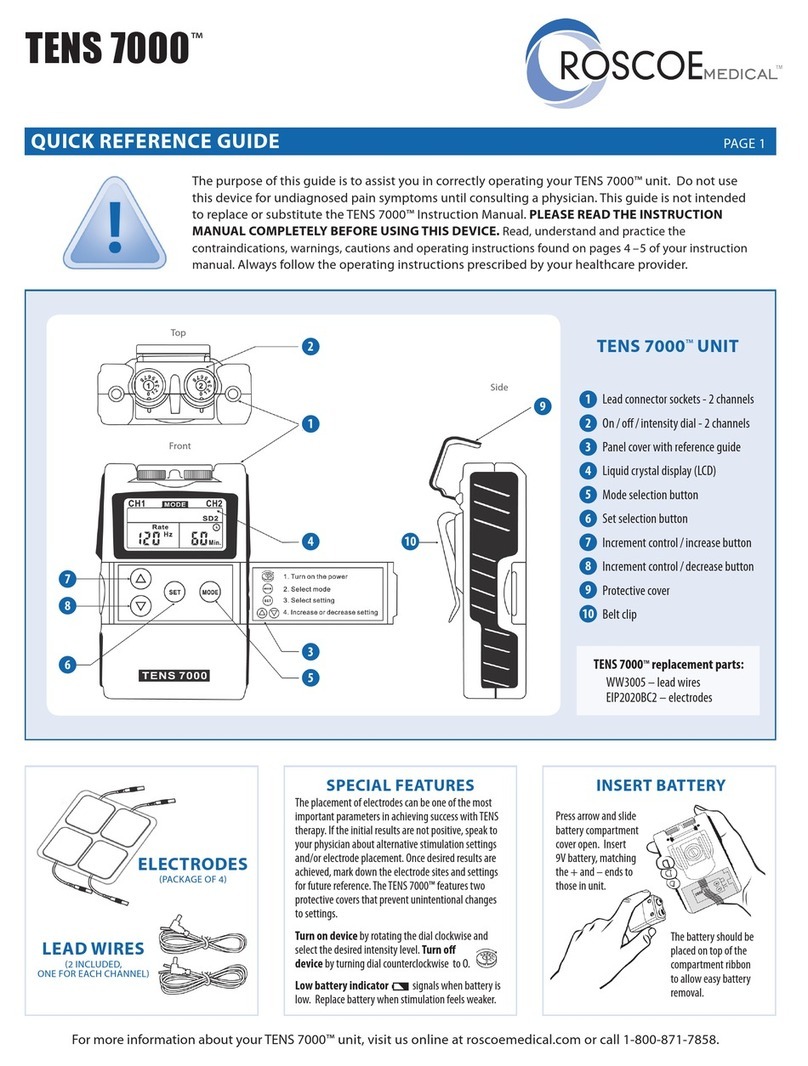
Fingertip
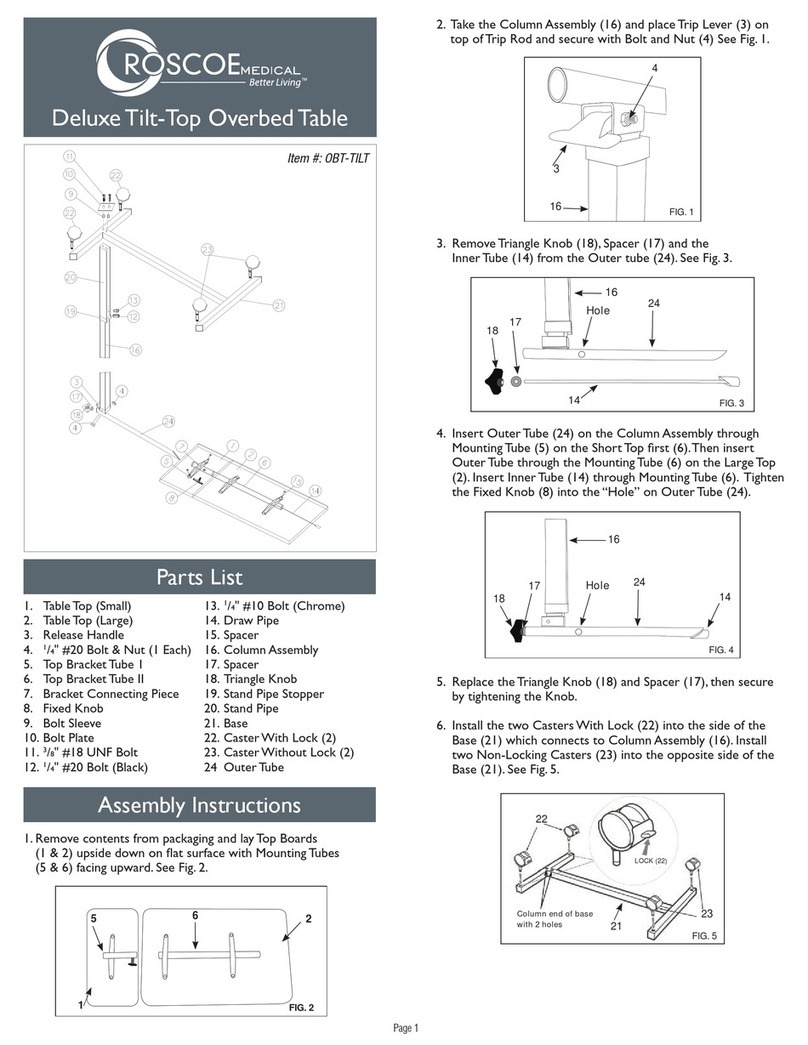
Pulse Oximeter
USER MANUAL
Ver2.0C2
General Description
Oxygen Saturation is a percentage of Oxyhemoglobin (HbO2) capacity, compounded with oxygen, by all combinative hemoglobin
(Hb) capacity in blood. In other words, it is consistency of Oxyhemoglobin in blood. It is a very important parameter for the
Respiratory Circulation System. Many respiratory diseases can result in oxygen saturation being lowered in human blood.
Additionally, the following factors can reduce oxygen saturation: Automatic regulation of organ dysfunction caused by Anesthesia,
Intensive Postoperative Trauma, injuries caused by some medical examinations. That situation might result in light-headedness,
asthenia, and vomiting. Therefore, it is very important to know the oxygen saturation of a patient so that doctors can find problems
in a timely manner.
The fingertip pulse oximeter features low power consumption, convenient operation and portability. Place one fingertip into the
photoelectric sensor for diagnosis and the pulse rate and oxygen saturation will appear on the display. It has been proven in clinical
experiments that it also features high precision and repeatability.
Measurement Principle
Principle of the oximeter is as follows: A mathematical formula is established making use of Lambert Beer Law according to
Spectrum Absorption Characteristics of Reductive hemoglobin(RHb) and Oxyhemoglobin (HbO2) in glow and near-infrared zones.
Operation principle of the instrument: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity
Pulse Scanning and Recording Technology, so that two beams of different wavelength of lights (660nm glow and 940nm near
infrared light) can be focused onto a human nail tip through a clamping finger-type sensor. A measured signal obtained by a
photosensitive element, will be shown on the oximeter’s display through process in electronic circuits and microprocessor shown on
the oximeter’s display through electronic circuits and a microprocessor.
Diagram of Operation Principle
1. Red and Infrared-ray Emission Tube
2. Red and Infrared-ray Receipt Tube
Precautions For Use
1 Before use, carefully read the manual.
2 Operation of the fingertip pulse oximeter may be affected by the use of an electrosurgical unit (ESU).
3 The fingertip pulse oximeter must be able to measure the pulse properly to obtain an accurate SpO2measurement. Verify
that nothing is hindering the pulse measurement before relying on the SpO2measurement.
4 Do not use the fingertip pulse oximeter in an MRI or CT environment.
5 Do not use the fingertip pulse oximeter in situations where alarms are required. The device has no alarms. It is not for
continuous monitoring.
6 Do not use the fingertip pulse oximeter in an explosive atmosphere.
7 The fingertip pulse oximeter is intended only as an adjunct in patient assessment. It must be used in conjunction with other
methods of assessing clinical signs and symptoms.
8 Check the pulse oximeter sensor application site every 4 hours to determine the positioning of the sensor and circulation
and skin sensitivity of the patient.
9 Do not sterilize the device using autoclaving, ethylene oxide sterilizing, or immersing the device in liquid. The device is not
intended for sterilization.
10 Follow local ordinances and recycling instructions regarding disposal or recycling of the device and device components,
including batteries.
11 This equipment complies with IEC 60601-1-2:2007 for electromagnetic compatibility for medical electrical equipment and/or
systems. However, because of the proliferation of radio-frequency transmitting equipment and other sources of electrical
noise in healthcare and other environments, it is possible that high levels of such interference due to close proximity or
strength of a source might disrupt the performance of this device.
12 Portable and mobile RF communications equipment can affect medical electrical equipment.
Rx only: “Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed practitioner.”
Inaccurate measurements may be caused by
1 Significant levels of dysfunctional hemoglobin (such as carbonyl - hemoglobin or methemoglobin);
2 Intravascular dyes such as indocyanine green or methylene blue;
3 High ambient light. Shield the sensor area if necessary;
4 Excessive patient movement;
5 High-frequency electrosurgical interference and defibrillators;
6 Venous pulsations;
7 Placement of a sensor on an extremity with a blood pressure cuff, arterial catheter, or intravascular line;
8 The patient has hypotension, severe vasoconstriction, severe anemia, or hypothermia;
9 The patient is in cardiac arrest or is in shock;
10 Fingernail polish or false fingernails;
11 Weak pulse quality (low perfusion);
12 Low hemoglobin;
Product Properties
1 Operation of the product is simple and convenient.
2 The product is small in volume, light in weight and convenient to carry.
3 Power consumption of the product is low and the two AAA batteries can be operated continuously for 30 hours.
4 A low voltage warning will be indicated when battery voltage is low and normal operation of the oximeter might be influenced.
5 The product will automatically power off when there is no signal for longer than 8 seconds.
Intended Use
Fingertip pulse oximeter is a portable non-invasive device intended for spot-checking of oxygen saturation of arterial hemoglobin
(SpO2) and pulse rate of adult and pediatric patient at home, and hospital (including clinical use in internist/surgery, Anesthesia,
intensive care etc). It is not for continuous monitoring.
The fingertip pulse oximeter requires no routine calibration or maintenance other than replacement of batteries.
Operation Instructions
1 Install two AAA batteries according to the Battery Installation instructions listed above in the right column.
2 Open the clamp as illustrated in the picture below.
3 Fully insert one fingertip into the silicone hole of the oximeter before releasing the clamp.
4 Press the switch button once on front panel.
5 Keep your finger still during measurement.
6 Read corresponding data from display screen.
7 Press the button again to toggle between six display modes.
After turning on the Oximeter, each time you press the power switch, the Oximeter will switch to another display mode.
There are 6 display modes shown as follows:
1. 2. 3.
4. 5. 6.
Holding the power switch for longer than one second, will adjust the brightness of the oximeter. There are 10 levels of brightness.
The default level is level four.
Front Panel
Patient pulse quality signals are indicated by bar graph. The bar is graded as 10 levels, if the strength is level 2 to 3, the pulse signal
is inadequate.
Product Accessories
1. One lanyard
2. Two AAA batteries
3. One instruction manual
Battery Installation
1. Install two AAA batteries into the battery compartment. Match the plus (+) and minus (-)
signs in the compartment. If the polarities are not matched, damage may be caused to
the oximeter.
2. Slide the battery door cover horizontally along the arrow shown as the picture.
Notes:
Install the batteries with the correct polarity. Incorrect placement may cause damage to
the bracket.
Please remove the batteries if the pulse oximeter will not be used for long periods of time.
Using the Lanyard
1. Thread thinner end of the lanyard through the hanging hole.
2. Thread thicker end of the lanyard through the threaded end before pulling it
tightly.
Warnings!
1. Keep the oximeter away from young children. Small items such as the battery door,
battery, and lanyard are choking hazards.
2. Do not hang the lanyard from the device’s electrical wire.
Maintenance and Storage
1. Replace the batteries in a timely manner when low voltage lamp is lighted.
2. Clean surface of the fingertip oximeter before it is used in diagnosis for patients.
3. Remove the batteries if the oximeter is not operated for a long time.
4. It is best to store the product in -20℃~+55℃and ≤93% humidity.
5. Keep in a dry place. Extreme moisture may affect oximeter lifetime and may cause damage.
6. Dispose of battery properly; follow any applicable local battery disposal laws.
Cleaning the fingertip pulse oximeter
Please use medical alcohol to clean the silicone touching the finger inside of oximeter with a soft cloth dampened with 70%
isopropyl alcohol. Also clean the being tested finger using alcohol before and after each test.
Do not pour or spray liquids onto the oximeter, and do not allow any liquid to enter any openings in the device. Allow the oximeter to
dry thoroughly before reuse.
A functional tester cannot be used to assess the accuracy of a pulse oximeter monitor or sensor. Clinical testing is used to establish
the SpO2accuracy. The measured arterial hemoglobin saturation value (SpO2) of the sensors is compared to arterial hemoglobin
oxygen (SaO2) value, determined from blood samples with a laboratory CO-oximeter. The accuracy of the sensors in comparison to
the CO-oximeter samples measured over the SpO2range of 70 –100%. Accuracy data is calculated using the
root-mean-squared (Arms value) for all subjects, per ISO 9919:2005, Medical Electrical Equipment–Particular requirements for the
basic safety and essential performance of pulse oximeter equipment for medical use.
Specifications
1. Display Type
OLED display
Data update time: <2s
2. SpO2
Display range: 0-99%
Measurement range: 70-99%
Accuracy: 70%-99%: ±3%; 0%~69% no definition
Resolution: 1%
3. Pulse Rate
Display range: 0~254BPM
Measure range: 30-235 BPM
Accuracy: 30~99bpm, ±2bpm; 100~235bpm, ±2%
Resolution: 1BPM
4. Probe LED Specifications
Wavelength Radiant Power
RED 660±2nm 1.8mW
IR 940±10nm 2.0mW
5. Power Requirements
Two AAA alkaline Batteries
Power consumption: Less than 40mA
Battery Life: Two AAA 1.5V, 600mAh alkaline batteries could be continuously operated as long as 30 hours.
It is equipped with a function switch, through which the oximeter can be powered off in case no finger is the oximeter longer than 8
seconds.
6. Outline Dimension
Length: 58mm
Width: 32mm
Height: 34mm
Weight: 50g (including two AAA batteries)
7. Environment Requirements
Operation Temperature: 5~40℃
Storage Temperature: -20~+55℃
Ambient Humidity: 15%~80% in operation
≤93% in storage
8. Equipment Response Time
As shown in the right figure.
RMI-POX4C