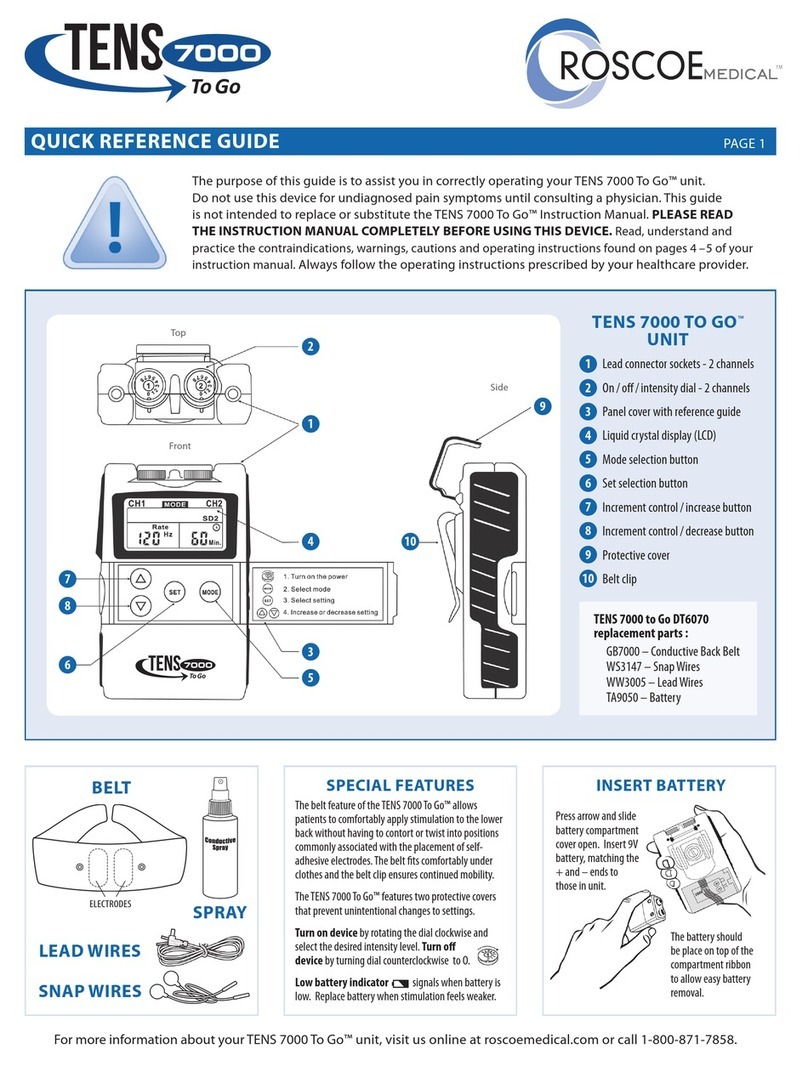
2.2
WARNING
5
1) United States Federal Law restricts this device to sale to, or on the order of,
a physician or licensed practitioner. This device should be used only under
the continued supervision of a physician or licensed practitioner.
2) Make certain the unit is electrically grounded by connecting only to a
grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
3) Care must be taken when operating this equipment around other equipment.
Potential electromagnetic or other interference could occur to this device or
to the other equipment. Try to minimize this interference by not using the
other equipment in conjunction with it.
4) Before administering any treatment to a patient you should become
acquainted with the operating procedures for each mode of treatment
available, as well as the indications, contraindications, warnings and
precautions. Consult other resources for additional information regarding
the application of electrotherapy.
5) To prevent electrical shock, disconnect the unit from the power source before
attempting any maintenance procedures.
6) The use of accessories, transducers and cables other than those specified,
with the exception of transducers and cables sold by the manufacturer as
replacement parts for internal components, may result in increased emissions
or decreased immunity of the device.
7) This device is not designed to be use in an MRI environment and should be
removed prior to MRI exposure.
8) Do not apply stimulation over the patient's neck because this could cause
severe muscle spasms resulting in closure of the airway, difficulty in breathing,
or adverse effects on heart rhythm or blood pressure.
9) Do not apply stimulation across the patient's chest, because the introduction
of electrical current into the chest may cause rhythm disturbances to the
patient's heart, which could be lethal.
10) Do not apply stimulation over open wounds or rashes, or over swollen, red,
infected, or inflamed areas or skin eruptions (e.g., phlebitis, thrombophlebitis,
varicose veins).
11) Do not apply stimulation over, or in proximity to, cancerous lesions.
12) Do not apply stimulation in the presence of electronic monitoring equipment
(e.g., cardiac monitors, ECG alarms), which may not operate properly when
the electrical stimulation device is in use.
13) Do not apply stimulation when the patient is in the bath or shower.
14) Do not apply stimulation while the patient is sleeping.
15) Do not apply stimulation while the patient is driving, operating machinery, or
during any activity in which electrical stimulation can put the patient at risk for
injury.
16) Consult with the patient's physician before using this device, because the
device may cause lethal rhythm disturbances to the heart insusceptible
individuals.
17) Apply stimulation only to normal, intact, clean, dry, healthy skin.
18) This device should not be used for symptomatic local pain relief unless
etiology is established or unless a pain syndrome has been diagnosed.
Quattro™ 2.5 DQ8450