Smith & Nephew RENASYS GO User manual
Other Smith & Nephew Medical Equipment manuals

Smith & Nephew
Smith & Nephew RENASYS GO User manual

Smith & Nephew
Smith & Nephew EXOGEN 4000+ User manual

Smith & Nephew
Smith & Nephew TRIGEN SURESHOT User manual

Smith & Nephew
Smith & Nephew LEGION Cones User manual

Smith & Nephew
Smith & Nephew JOURNEY II BCS User manual

Smith & Nephew
Smith & Nephew RENASYS EDGE User manual

Smith & Nephew
Smith & Nephew POLARSTEM 75004650 Configuration guide

Smith & Nephew
Smith & Nephew SPIDER2 User manual
Smith & Nephew
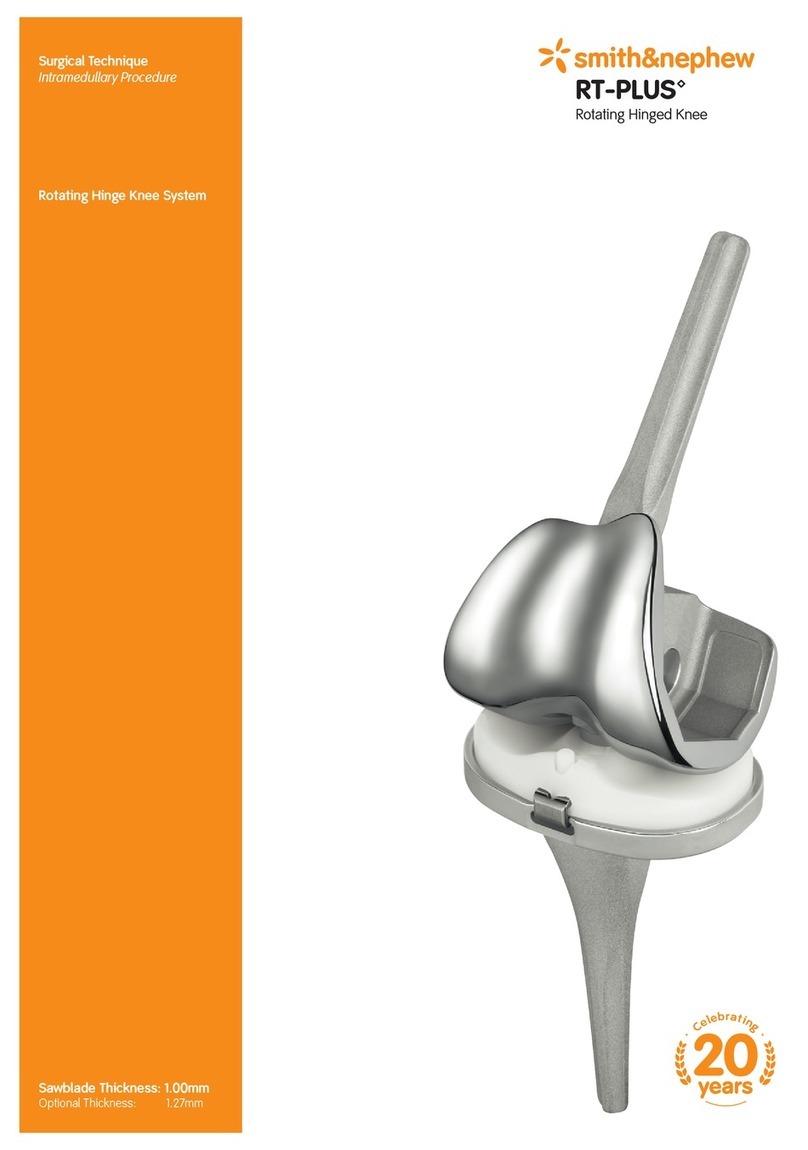
Smith & Nephew RT-PLUS User manual
Smith & Nephew
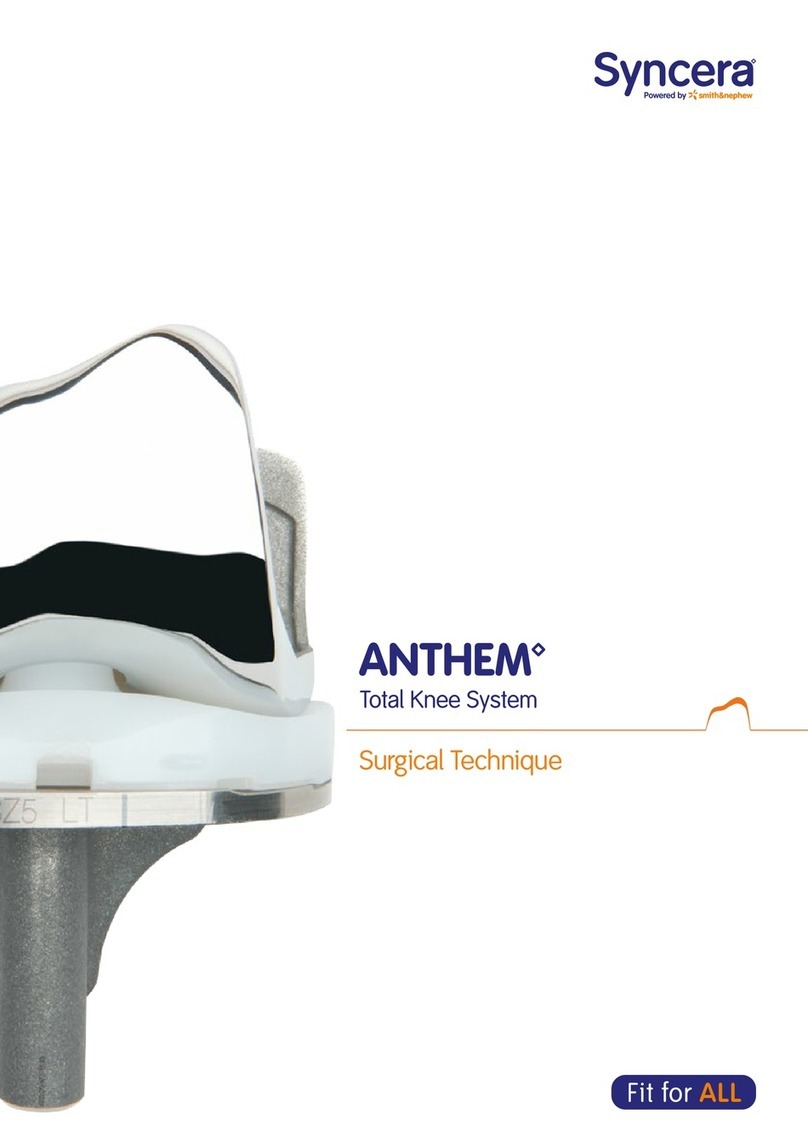
Smith & Nephew Syncera Anthem User manual

Smith & Nephew
Smith & Nephew Dyonics 300XL Installation and operating instructions

Smith & Nephew
Smith & Nephew PICO User manual

Smith & Nephew
Smith & Nephew RENASYS EDGE Instruction Manual

Smith & Nephew
Smith & Nephew SPIDER2 User manual

Smith & Nephew
Smith & Nephew RENASYS GO User manual

Smith & Nephew
Smith & Nephew REDAPT User manual

Smith & Nephew
Smith & Nephew RENASYS GO User manual

Smith & Nephew
Smith & Nephew RT-PLUS User manual

Smith & Nephew
Smith & Nephew TC-PLUS Revision User manual

Smith & Nephew
Smith & Nephew ZUK User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual




















