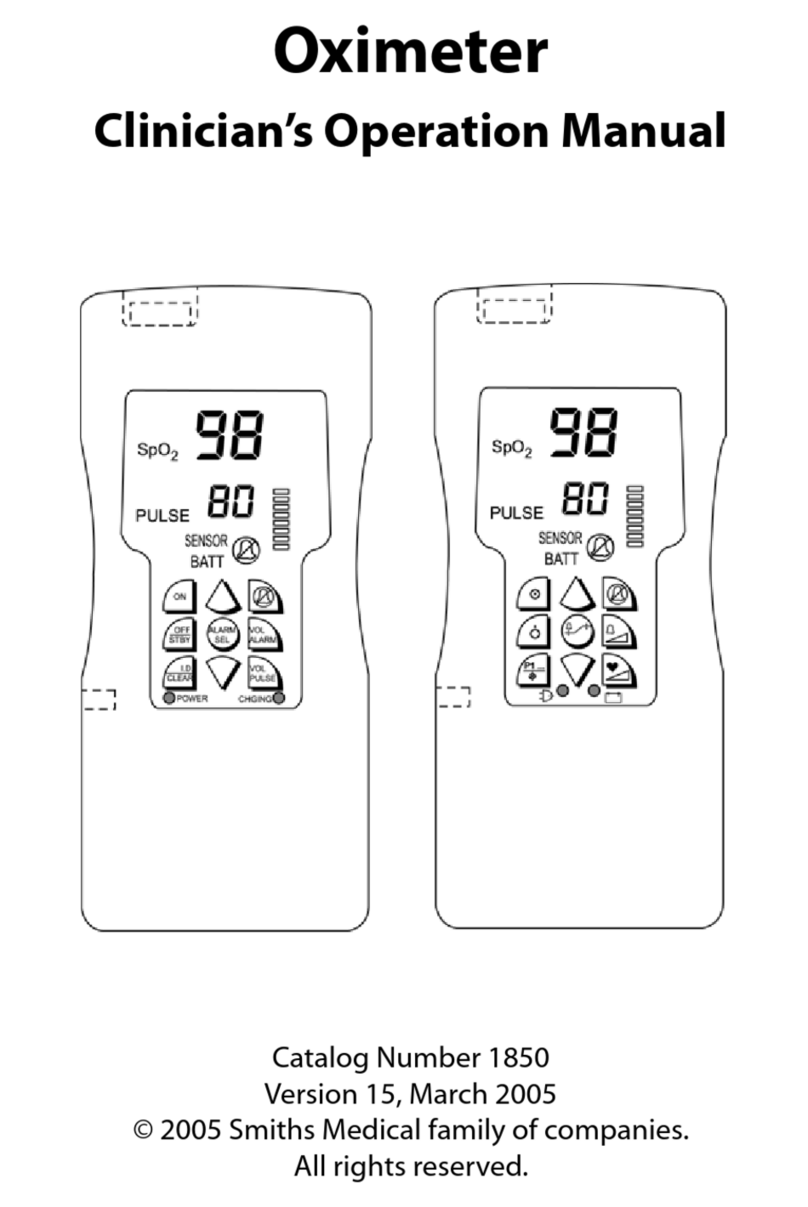
Smiths Medical ASD, Inc.
Keene, NH 03431, USA
Phone: +1-603-352-3812
Toll-Free USA 1-800-258-5361
www.smiths-medical.com
Find your local contact information at: www.smiths-medical.com/customer-support
Smiths Medical is part of the global technology business Smiths Group plc. acapella and the Portex and the Smiths Medical design marks are trademarks of Smiths Medical. The symbol ® indicates the
trademark is registered in the U.S. Patent and Trademark Office and certain other countries. All other names and marks mentioned are the trademarks or service marks of their respective owners.
©2016 Smiths Medical. All rights reserved. RE194317EN-072016
Ref acapella®
DM (Blue)
system
Product Code
acapella®
DH (Green)
system
Code
Quantity
Per Case
Mouthpiece 21-1015 21-1530 10
Pediatric mask 21-3015 21-3530 1
Medium Mask 21-5015 21-5530 1
Large mask 21-7015 21-7530 1
Accessory
Products
Product
Code
Quantity
Per Case
Pressure Port 20-0010 10
TheraPEP®
pressure indicator,
tubing, and
pressure port
20-0022 10
MKEECA-0101
PRODUCT(S) DESCRIBED MAY NOT BE LICENSED OR AVAILABLE FOR SALE IN CANADA AND OTHER COUNTRIES
2 6
5. PRECAUTIONS
5.1 Bleach is not recommended for use on the
acapella®. It may deteriorate the nickel plated
mechanism located in the interior of the device.
5.2 DO NOT MICROWAVE.
The metal and magnet might ignite.
5.3 It is the responsibility of the user to
ensure all sterility verification(s).
5.4 Visually inspect the device to ensure
it is free of contamination and foreign objects.
5.5 Verify all connections are secure.
6. SUGGESTED INSTRUCTIONS FOR USE
6.1 Initial Settings
6.1.1 If this is the first use of the acapella®systems,
ensure the frequency adjustment dial is turned
counterclockwise to its lowest frequency-
resistance setting.
(See Figure 2)
6.1.2 Instruct the patient to relax while performing
diaphragmatic breathing and inspiring a volume
of air larger than normal tidal volume
(but not to total lung capacity).
6.1.3 Direct the patient to exhale to Functional
Residual Capacity (FRC) actively, but not
forcefully, through the device.
6.1.4 The patient should be able to exhale for
3-4 seconds while the device vibrates. If
the patient cannot maintain an exhalation
for this length of time, adjust the dial
clockwise (See Figure 2). Clockwise
adjustment increases the resistance of
the vibrating orifice, which will allow the
patient to exhale at a lower flow-rate.
6.1.5 Selection of the proper resistance range
produces the desired inspiratory to
expiratory (I:E) ratio of 1:3 to 1:4.
Note: If the desired resistance and I:E
ratio cannot be achieved, consider using
an acapella®device designed for
alternate flow ranges.
6.1.6 Once the proper range has been
identified, the patient may be instructed
to exhale harder or softer, or dial
adjustments may be made to optimize
the response the user “feels” from the
vibratory pressure.
6.1.7 Several uses may be needed to ensure
that individual patient needs are being met.
6.2 Procedure for the User
6.2.1 Ensure the adjustment dial is set to the
correct range as identified by your clinician.
6.2.2 Sit with your elbows resting comfortably on
the table.
6.2.3 Place the mouthpiece lightly in your mouth.
• •Be sure to maintain a tight seal on
the mouthpiece during exhalation.
• •Your clinician may recommend the
use of a nose clip, if necessary.
• •If using a mask, apply the mask tightly but
comfortably over nose and mouth.
6.2.4 Breathe from the diaphragm, as directed
by your clinician, taking in a larger than
normal breath, but not filling your lungs
to capacity.
6.2.5 Hold your breath for 2-3 seconds.
6.2.6 Exhale actively, but not forcefully,
through the device. Exhalation should
last approximately 3 to 4 times longer
than inhalation.
6.2.7 Perform 10-20 PEP breaths as
recommended by your clinician.
6.2.8 Remove the mouthpiece (mask) and perform
2-3 “huff” coughs to raise secretions as
needed. Your clinician may direct you on
proper cough technique.
6.2.9 Repeat steps 6.2.2 to 6.2.7 as prescribed.
Note: See nebulizer set-up section.
6.3 Set-up Nebulizer
6.3.1 Review the diagrams contained with
the devices.
6.3.2 A possible nebulizer and acapella®system set
up is reflected below. (See Figure 3)
6.3.3 Follow set-up instructions for each device.
6.3.4 Follow cleaning instructions contained
with each device.
6.3.5 Inspect device(s) on a routine basis
to ensure proper use and function.
6.3.6 If damaged, do not use.
6.3.7 Verify all connections are secure.
6.4 Set up pressure gauge/indicator:
(See Figure 4)
6.4.1 Place the pressure gauge/indicator
(Smiths 20-0010) between the
mouthpiece and the device.
6.4.2 Verify all connections are secure.
7. CLEANING AND
DISINFECTING INSTRUCTIONS
Precaution: Bleach is not recommended
for use on the acapella®system. It may
deteriorate the nickel plated mechanism
located in the interior of the device.
7.1 Cleaning: This should be done prior to
disinfecting (7.2) As per the Cystic Fibrosis
Foundation’s cleaning and disinfecting
guidelines entitled, “Respiratory, Stopping
the Spread of Germs” 2008, below are the
guidelines for the acapella®system.
Cleaning with Liquid Dish Detergent:
As needed, detach the mouthpiece (mask) then
soak the device and mouthpiece in warm, soapy
water as required to remove visible
contaminants. Use a liquid dish detergent
(Dawn®or equivalent), mixing two (2)
tablespoons of detergent per one (1) gallon
water. Rinse thoroughly with sterile water, and
allow parts to air dry. Drain the device by placing
it in a normal resting position. (See Figure 1)
7.2 Disinfecting
• •Alcohol –Soak five (5) minutes, twice daily. The
acapella®system is compatible with 70%
isopropyl alcohol. Rinse with sterile water. You
can make water sterile by boiling for five
(5) minutes.
• •Hydrogen Peroxide – Soak in 3% hydrogen
peroxide for 30 minutes Rinse with sterile water.
You can make water sterile by boiling for five
(5) minutes.
• •Glutaraldehydes (Cidex®or equivalent) – The
acapella®system will maintain its integrity
using cold sterilizing solutions such as
glutaraldehydes.
8. DISPOSAL
Dispose of the acapella®system in a safe
manner according to Federal/State/Local
regulations and guidelines for disposal of
contaminated medical waste.
9. ESTIMATED DEVICE LIFETIME
Assuming the manufacturer recommended
cleaning protocol is followed, the acapella®
system should have a useful life of six (6)
months under normal and customary usage.
The six (6) month usage duration is measured
from the date of initial use.
f ~ : ; ‚ < = J
Caution • Latex Free • Non-sterile
Do not use if package is damaged
[www.smiths-medical.com/phthalates]
• Catalog Number • Batch Code
Date of Manufacture.
Caution: Federal (U.S.A.) law restricts this device
to sale by or on the order of a physician.
SINGLE PATIENT USE