Steris VHP X10 User manual




















Table of contents
Other Steris Laboratory Equipment manuals

Steris
Steris AMSCO 30 Series User manual

Steris
Steris SYSTEM 1E User manual

Steris
Steris V-PRO maX 2 User manual

Steris
Steris SYSTEM 1 endo User manual

Steris
Steris AMSCO 600 Medium 639V-3 User manual

Steris
Steris SYSTEM 1 endo User manual

Steris
Steris Basil 3500 User manual

Steris
Steris V-PRO 60 User manual

Steris
Steris Amsco 600 ALUS User manual

Steris
Steris AMSCO 400 Series User manual

Steris
Steris Basil 9502 User manual

Steris
Steris Reliance 5000 Series User manual

Steris
Steris AMSCO 1215 User manual

Steris
Steris VHP MD140X User manual

Steris
Steris Basil 1100 User manual

Steris
Steris Basil 6000 User manual

Steris
Steris AMSCO 2532 User manual

Steris
Steris Reliance EPS Endoscope Processing System User manual

Steris
Steris Basil 4700 User manual

Steris
Steris Reliance 1024 User manual
Popular Laboratory Equipment manuals by other brands

Fisher Scientific
Fisher Scientific Fisherbrand accuSpin Micro Series instruction manual

Energenics
Energenics UV-MAX Installation & operation manual
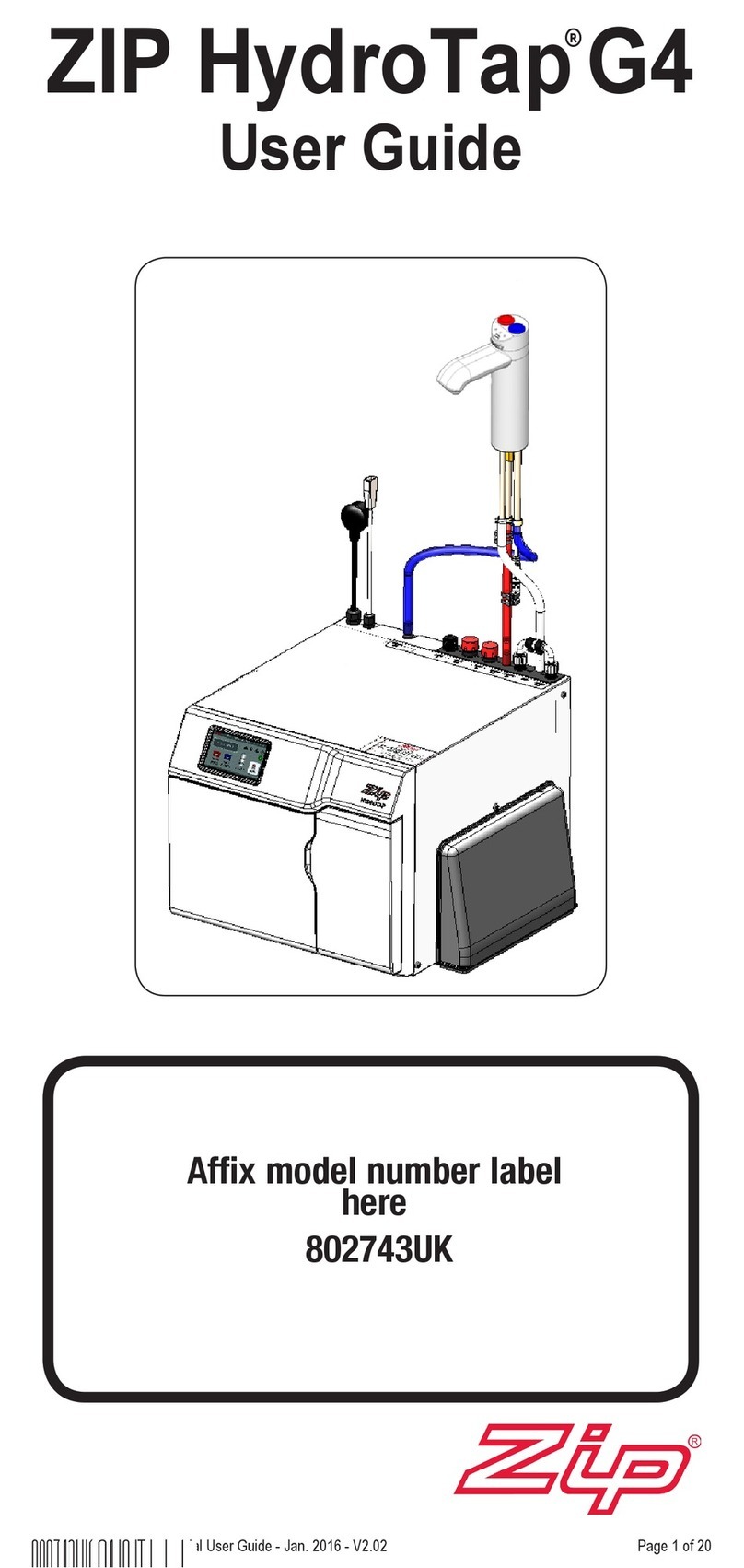
Zip
Zip HydroTap G4 user guide

THORLABS
THORLABS KURIOS-WB1 user guide

Daihan Scientific
Daihan Scientific MaXircu WHR user manual

Rongbai Precision Pump
Rongbai Precision Pump BT300S Operation manual

PRO Scientific
PRO Scientific Bio-Gen PRO200 operating manual

Telemark Cryogenics
Telemark Cryogenics 1200 instruction manual

Hach
Hach PHC745 user manual

Memmert
Memmert HPP 108 operating instructions

Oxford Instruments
Oxford Instruments ANDOR Kymera 328i Series user guide

Luxul
Luxul XPE-2500 Quick install guide





