Storz OR1 FUSION WO300 User manual

Instructions for use
KARL STORZ OR1 FUSION®, Rel. 1.4.2
WO300
en

Copyright ©
All product illustrations, product descriptions, and texts are the intellectual property of
KARLSTORZSE&Co.KG.
Their use and reproduction by third parties require the express approval of
KARLSTORZSE&Co.KG.
All rights reserved.
02-2021

Table of contents
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 3
Table of contents
1 General information.......................................................................................................................................5
1.1 Read the instructions for use.................................................................................................................... 5
1.2 Other applicable documents.....................................................................................................................5
1.3 Description of warning messages.............................................................................................................5
2 Normal use....................................................................................................................................................6
2.1 Intended use .............................................................................................................................................6
2.2 Indications.................................................................................................................................................6
2.3 Contraindications......................................................................................................................................6
2.4 Patient groups...........................................................................................................................................6
2.5 Target user populations ............................................................................................................................ 6
3 Safety ............................................................................................................................................................7
3.1 Serious incidents ......................................................................................................................................7
3.2 Correct reprocessing ................................................................................................................................7
3.3 Correct handling .......................................................................................................................................7
3.4 Damaged products ...................................................................................................................................7
3.5 Combination with other components .......................................................................................................8
3.6 Dangers from electrical current.................................................................................................................8
3.7 Damage due to ingress of liquid in electrical components....................................................................... 9
3.8 Risk of explosion.......................................................................................................................................9
3.9 Observing ambient conditions ..................................................................................................................9
3.10 Electromagnetic interference ..................................................................................................................10
3.11 Image display and transmission .............................................................................................................10
3.12 Data loss .................................................................................................................................................11
4 Product description ....................................................................................................................................12
4.1 Description of function............................................................................................................................12
4.2 Product overview ....................................................................................................................................12
4.3 Technical data.........................................................................................................................................13
4.4 Symbols employed .................................................................................................................................14
4.4.1 Symbols on the packaging .......................................................................................................... 14
4.4.2 Symbols on the product .............................................................................................................. 15
4.4.3 Symbols on the type plate........................................................................................................... 16
4.5 Ambient conditions .................................................................................................................................16
5 Preparation..................................................................................................................................................17
5.1 Unpacking the product ...........................................................................................................................17
5.2 Setting up the device ..............................................................................................................................17
5.3 Connecting the product ..........................................................................................................................17
6 Operation ....................................................................................................................................................18
6.1 Switching on the product........................................................................................................................18
6.2 User interface..........................................................................................................................................18
6.2.1 Symbols on the user interface..................................................................................................... 19
6.3 Onscreen keyboard.................................................................................................................................20
6.4 Volume control........................................................................................................................................ 20
6.5 Presets ....................................................................................................................................................21
6.5.1 Creating a preset ......................................................................................................................... 21
6.5.2 Using the preset .......................................................................................................................... 23
6.5.3 Searching for a preset ................................................................................................................. 24
6.6 "Current procedure" application area.....................................................................................................25
6.6.1 Patient.......................................................................................................................................... 25
6.6.2 Checklist ...................................................................................................................................... 31
6.6.3 Capture ........................................................................................................................................ 33
6.6.4 Edit............................................................................................................................................... 38

Table of contents
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 4
6.6.5 Finish ........................................................................................................................................... 40
6.7 "Control" application area.......................................................................................................................41
6.7.1 Anesthesia Safety ........................................................................................................................ 42
6.7.2 Routing ........................................................................................................................................ 43
6.7.3 Controlling the room camera....................................................................................................... 51
6.7.4 Medical Device Control – SCB .................................................................................................... 52
6.7.5 Entertainment .............................................................................................................................. 53
6.7.6 Light............................................................................................................................................. 54
6.7.7 Room Control .............................................................................................................................. 55
6.8 "Previous procedures" application area ................................................................................................. 56
6.8.1 Filing cabinet................................................................................................................................ 56
6.8.2 Open tasks................................................................................................................................... 59
6.9 "Communication" application area .........................................................................................................60
6.9.1 Access status .............................................................................................................................. 61
6.9.2 Streaming .................................................................................................................................... 63
6.9.3 Phone........................................................................................................................................... 64
6.9.4 Unified Communication ............................................................................................................... 66
6.9.5 Video conference......................................................................................................................... 73
6.9.6 Room connection ........................................................................................................................ 79
6.10 Switching off the product .......................................................................................................................85
7 Maintenance, servicing, repairs, and disposal............................................................................................86
7.1 Maintenance ...........................................................................................................................................86
7.2 Repairing devices ...................................................................................................................................86
7.3 Disposing of the product ........................................................................................................................86
8 Accessories and spare parts ......................................................................................................................87
8.1 Recommended accessories ...................................................................................................................87
9 Electromagnetic compatibility.....................................................................................................................88
9.1 General information on the operating environment ................................................................................ 88
9.2 Cables ..................................................................................................................................................... 88
9.3 Table 1 – Compliance level for immunity tests ....................................................................................... 89
9.4 Table 2 – Test levels for proximity fields from HF wireless communications equipment ....................... 90
9.5 Table 3 – Test levels for radiated and conducted immunity tests .......................................................... 91
9.6 Table 4 – Emission class and group .......................................................................................................92
9.7 Table 5 – Recommended separation distances between portable and mobile HF communications
equipment and the product ....................................................................................................................92
9.8 Regulatory information on telecommunications ..................................................................................... 93
9.8.1 USA.............................................................................................................................................. 93
9.8.2 Canada ........................................................................................................................................ 93
9.8.3 European Union ........................................................................................................................... 93
10 Subsidiaries.................................................................................................................................................94

General information
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 5
1 General information
1.1 Read the instructions for use
If the instructions for use are not followed, patients, users, or third parties may be injured. In
addition, the product may be damaged.
1. Read the instructions for use of the product carefully and follow them completely.
2. Keep the instructions for use clearly visible next to the product.
3. Observe the instructions for use of products used in combination.
It is recommended to check the suitability of the products for the planned procedure prior to
use.
1.2 Other applicable documents
The following documents are a part of the product and must be observed:
– instructions for use
– installation instructions
– reprocessing instructions
1.3 Description of warning messages
To prevent any injury to persons or damage to property, the warnings and safety notes in the
instructions for use must be observed. The warning messages describe the following levels of
danger.
WARNING
WARNING
Designates a possible imminent risk. If this is not avoided, it could lead to death or serious
injuries.
CAUTION
CAUTION
Designates a possible imminent risk. If this is not avoided, it could lead to minor injuries.
ATTENTION
ATTENTION
Designates a possibly harmful situation. If this is not avoided, the products could be damaged.

Normal use
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 6
2 Normal use
2.1 Intended use
The OR1 FUSION CONTROL® is designed for use in operation rooms by qualified personnel.
The OR1 FUSION CONTROL® is an appliance (comprising hardware and software) for the
documentation of audiovisual data and patient data during diagnostic and therapeutic
procedures. It allows the operation to be recorded and described for documentation purposes.
The audiovisual data that is recorded and forwarded using the OR1 FUSION CONTROL® is for
examination and information purposes, and not primarily for making diagnoses. The recorded
audiovisual data is not intended to be shown intraoperatively on the operation monitor.
2.2 Indications
Routing of audio, video, and control signals from signal sources to signal destinations in the
case of medical procedures.
2.3 Contraindications
Use is contraindicated if, in the opinion of the responsible physician, the device is not
compatible with successful completion of the planned procedure due to its technical design.
2.4 Patient groups
There are no restrictions in terms of patient groups for this product.
The product does not come into direct contact with the patient.
2.5 Target user populations
The medical device may only be used by doctors and medical assistants with a relevant
specialist qualification.

Safety
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 7
3 Safety
3.1 Serious incidents
According to the Medical Device Regulation (MDR), a “serious incident” includes incidents that
directly or indirectly had, could have had, or could have any of the following consequences
(MDR, Art.2, No.65[1]):
– Death of a patient, user, or another person
– Temporary or permanent serious deterioration in the medical condition of a patient, user,
or another person
– A serious threat to public health
The manufacturer and appropriate authority must be notified of all serious incidents.
3.2 Correct reprocessing
Incorrectly reprocessed products expose patients, users, and third parties to a risk of infection.
Reprocess the product before use.
A validated reprocessing procedure must be followed and the product must be
reprocessed in line with the reprocessing instructions.
3.3 Correct handling
If the product is not handled correctly, patients, users, and third parties may be injured.
Only persons with the necessary medical qualification and who are acquainted with the
application of the product may work with it.
Do not convert or modify the product.
3.4 Damaged products
Damaged products can result in injury to patients, users, and third parties.
Before each use, check all components of the product as well as the entire system with all
connected devices for damage.
Do not use damaged products.
Have defective products checked by or by companies authorized by .
Have regular safety inspections performed by or by companies authorized by .
The product may fail during use. In this case, image transmission will be interrupted. The
patient may be injured.
Always have a backup system (direct line between endoscopic camera and monitor) ready.
In case of a failure or malfunction, cease work immediately and switch to a backup
system.
Prior to each operation, make sure that the personnel are familiar with the procedure for
switching over to the backup system.
Devices that emit excessively high interference radiation due to a defect can impact monitors
or other devices.
Switch off the defective device.
With an OR1 ™ InWall Solution, the temperature and ventilation around the product are
continuously monitored. If the temperature around the product exceeds a certain value, an
error message is issued.

Safety
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 8
Prepare to use the direct line between the endoscopic camera and monitor, as the product
can fail as a result of excessively high temperatures.
Inforn the service.
Before each intervention, ensure that personnel are trained in dealing with the direct line
(substitute system).
3.5 Combination with other components
The use of unauthorized devices and components or unauthorized changes to the product can
lead to malfunctions and injuries.
Additional devices connected to electrical medical equipment must comply with the relevant
IEC or ISO standards. Furthermore, all configurations must comply with the requirements for
medical electrical systems (see IEC 60601-1-1 or clause 16 of the 3rd edition of IEC 60601-1).
Any person who connects additional devices or components to the product changes the
medical electrical system and is therefore responsible for ensuring that it complies with the
requirements for medical electrical systems.
Only combine the product with devices and components that are approved for joint use by
the manufacturer.
Only use approved accessories.
Observe the instruction manuals and interface specifications of the devices and
components used in combination.
Only use devices and components that have standardized interfaces and do not breach
the intended use of the product.
Only use devices that can be displayed in the monitor as a "playback device."
Perform an equipment test before use.
Comply with national and local regulations.
Only make changes to the product if these changes are approved by .
3.6 Dangers from electrical current
An improper power supply may cause an electric shock and injure patients, users, or third
parties. All electrical installations of the operation room in which the product is connected and
used must meet the applicable IEC standards. Input and output equipment connected to the
product must comply with IEC60601-1.
Have the device installed and put into service by authorized and trained electricians of or
by companies authorized by .
Use either the power cord supplied by or a power cord which has the same properties and
which bears a national mark of conformity.
The product may only be operated with the line voltage stated on the rating plate.
Position the product appropriately so that the power cord can be unplugged at any time.
The product is only voltage-free when the mains plug has been disconnected.
Ensure potential equalization according to the applicable national rules and regulations.
To ensure reliable protective earth grounding, connect the product to a properly installed
socket that is approved for use in the operation room. Routinely inspect the electrical plug
and cord and do not use if the inspection reveals damage.
Connect the product to a power supply with protective conductor.
If several devices are connected to the product, note the maximum leakage current.
Do not use any additional multiple socket outlets or extension cables.
Do not use freely accessible multiple socket outlets.
Multiple socket outlets must not be placed on the floor.

Safety
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 9
In the case of electrical products, individual components or the product itself may be live. Live
parts can cause electric shocks in the event of contact and injure patients, users, and third
parties.
Do not open the product.
Have servicing carried out by or a company authorized by . Failure to observe this will void
the guarantee.
Do not touch the output jacks of the product and the patient at the same time during use.
Always pull out the mains plug before carrying out any cleaning and maintenance work.
Disconnect the power plug before opening the housing.
3.7 Damage due to ingress of liquid in electrical
components
In the case of electrical products, individual components or the product itself may be live.
Liquid ingress into an electrical product may result in a short circuit or an unintentional transfer
of current. The product is damaged as a result and patients, users and third parties may be
injured.
Do not store liquids near the product or on the product.
If liquid has entered the product, pull out the plug and allow the product to dry completely.
3.8 Risk of explosion
The product can generate sparks, which cause combustible or flammable gases and liquids to
ignite or explode. This may cause injuries to patients, users, and third parties.
The product must not be operated in oxygenated environments.
Do not operate the product in explosive atmospheres.
Do not operate the product in environments with combustible gases such as inhalation
anesthetics and mixtures thereof. Observe the hazard zones:
Only connect or disconnect the power plug to or from the power supply outside explosive
atmospheres.
Combustible and flammable gases must be allowed to escape, be extracted, or be
displaced with CO2 before use.
Combustible and flammable liquids must be allowed to vaporize or be extracted before
use.
Only start the application when combustible or flammable gases and liquids are no longer
present.
3.9 Observing ambient conditions
If the product is used in an unsuitable environment, patients, users, and third parties may be
injured.
Safety
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 10
The electrical installations in the operating room in which the device is connected and
operated must comply with the applicable IEC standards.
Always operate the product in the prescribed ambient conditions.
Position the product so that there is sufficient free space for air circulation.
Install the product and its components out of reach of patients.
3.10 Electromagnetic interference
Medical electrical products are subject to special precautions regarding electromagnetic
compatibility and must be installed and commissioned according to the tables on
electromagnetic compatibility. If other products (e.g., for MRT, CT, diathermy, electrocautery,
or HFID) emit electromagnetic radiation, the function of the product may be disturbed. High-
frequency communication equipment can affect electrical medical products and impair their
performance.
Do not use the product in the vicinity of a magnetic resonance tomograph (MRT).
Do not use the product next to or together with other devices. If such use is required,
monitor the product and the other devices, and follow the relevant instructions for use in
the event of malfunctions.
Portable RF communications equipment including peripheral devices (e.g., antenna cables
and external antennas) should be used no closer than 30cm from the product, including
cables specified by the manufacturer.
Observe the information on electromagnetic compatibility; see chapter Electromagnetic
compatibility [p.88].
In case of uncertainties, seek expert advice from .
Before use, a clinical/biomedical engineer or an EMC specialist should carry out an ad-hoc
test of the electromagnetic radiation.
To prevent increased electromagnetic emissions or reduced electromagnetic immunity of
the product, only use accessories, transducers, and cables recommended or supplied by
the manufacturer.
In order to avoid exposing patients, users, or third parties to harmful electromagnetic
interference, the product must not be operated outside of its intended EMC environment.
Furthermore, the product must not be operated if the housing, cable, or electromagnetic
shielding equipment is damaged.
3.11 Image display and transmission
Recordings and image preview are not intended to be used for diagnostic or therapeutic
purposes.
The following data may only be used for documentation purposes:
– Image information in printed and electronically recorded form.
– Image information that originates from or was recorded by equipment not manufactured
by .
Always use the monitor of the connected device for diagnostic images. Signals in 3D will
only display correctly on suitable display equipment.
Image data may be lost if the device is switched off while processing patient data.
Only switch off the product using the rocker switch.
Connecting non-compatible signal types to the patch panel in the OR can cause image
interference and malfunctioning.
Only connect permitted signal types.
Perform equipment tests before use.

Safety
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 11
3.12 Data loss
Data may be lost when working on the software and hardware or on the hard disk, e.g., during
a system recovery.
Back up existing data before formatting the hard disk.
Only transfer encrypted data.
Ensure that data storage is finished and only then remove an external storage medium.
Perform a system test which verifies perfect functioning after adding new software or
hardware.
Shut down the software via the user interface or by briefly pressing the rocker switch.
Product description
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 12
4 Product description
4.1 Description of function
is designed for use in the operating rooms of clinics and hospitals.
The product is used to aid the implementation and documentation of medical procedures
aimed at examining and treating patients.
The following can be performed from the central touchscreen:
– Control non-medical devices.
– Transfer and display video and audio signals from the integrated signal sources to the
integrated peripheral devices.
– Record, save, edit, forward, and print medical examinations and procedures in the form
of stills, video sequences, and audio sequences.
4.2 Product overview
WARNING
Electric shock!
There is a voltage of 12V / 2A at the ‘Smart Screen’ socket.
Do not touch the socket during operation.
Only connect or remove the cable when the device is switched off.
12345
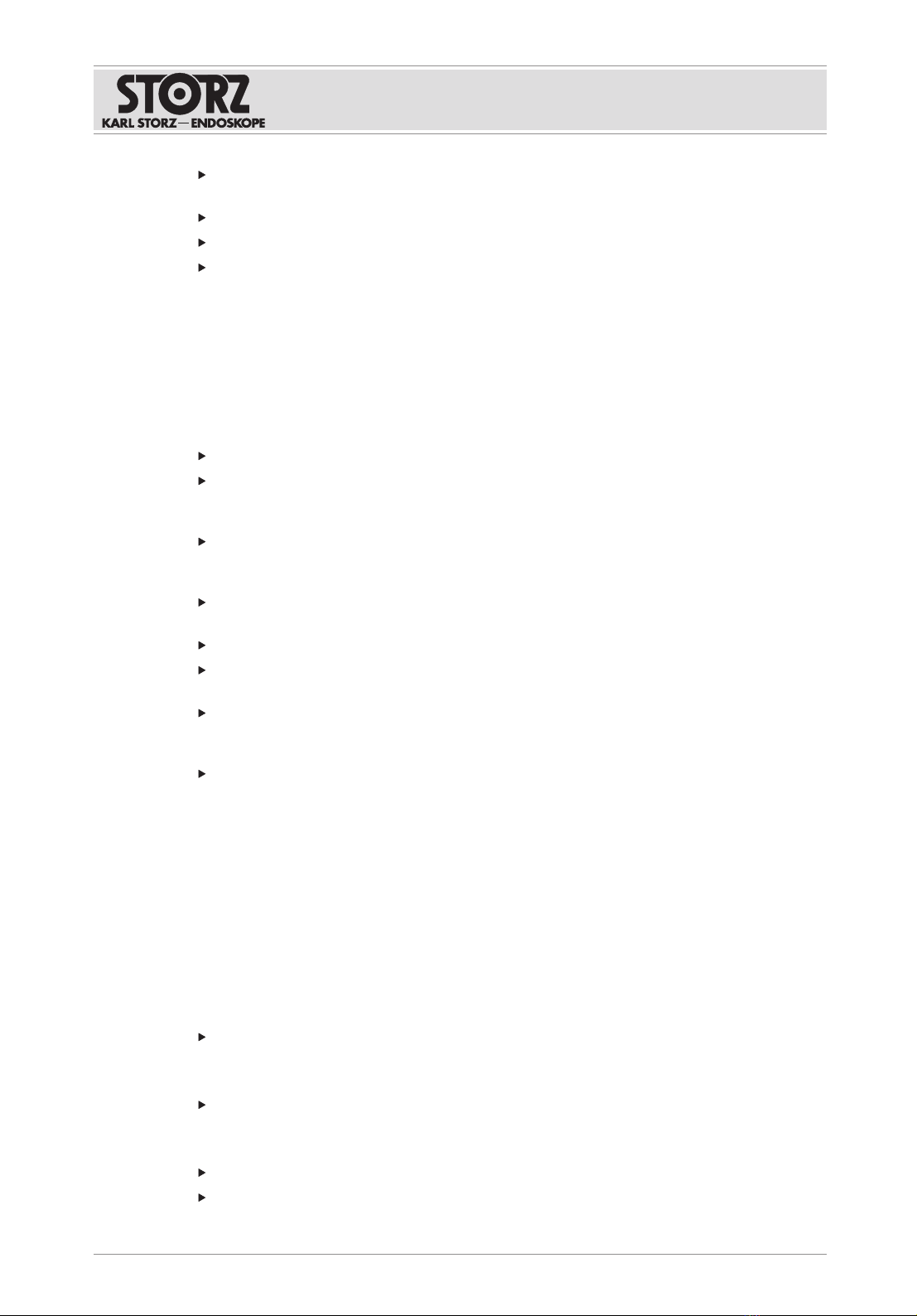
OR1 FUSION CONTROL® front view
1Rocker switch ON/OFF
Standby and operating mode
4 "Red" LED for hard disk access
2 "Yellow" standby LED 5 USB port
3 "Green" operating LED

Product description
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 13
123568
9
10
11
121314151617
47
18
OR1 FUSION CONTROL® rear view
1 Power connection 10 On Air Light (Phoenix 2-pin)
2 Potential equalization connection 11 Mini jack (audio IN/OUT)
3 Serial interface (DE 9) 12 4x USB 3.0
4 4x 3.5 mini jack (camera head buttons) 13 2x LAN (RJ-45)
5 Lemo 5-pin (foot pedal) 14 Monitor (DVI)
6 Serial interfaces, smart screen (D-Sub
DE 9)
15 2x monitor (digital port)
7 Serial interface (DE 9) 16 4x USB 2.0
8 2x 10G fiber-optic interface 17 Keyboard (PS/2)
9 Remote switch (Phoenix 4-pin) 18 Mouse (PS/2)
4.3 Technical data
Description Value
Power supply
Operating voltage 100–240V
Operating frequency 50–60Hz
Maximum current consumption 5–2A
Electrical protection class I
Degree of protection acc. to IEC 60259 IPX0
Housing
Dimensions (LxHxW) 355 x 74.5 x 305mm
Weight 6kg
System
Hard disk capacity 2 TB
Random Access Memory (RAM) 16 GB
Product description
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 14
Description Value
Operating system Windows® 10 Embedded
Operating mode Continuous operation
4.4 Symbols employed
4.4.1 Symbols on the packaging
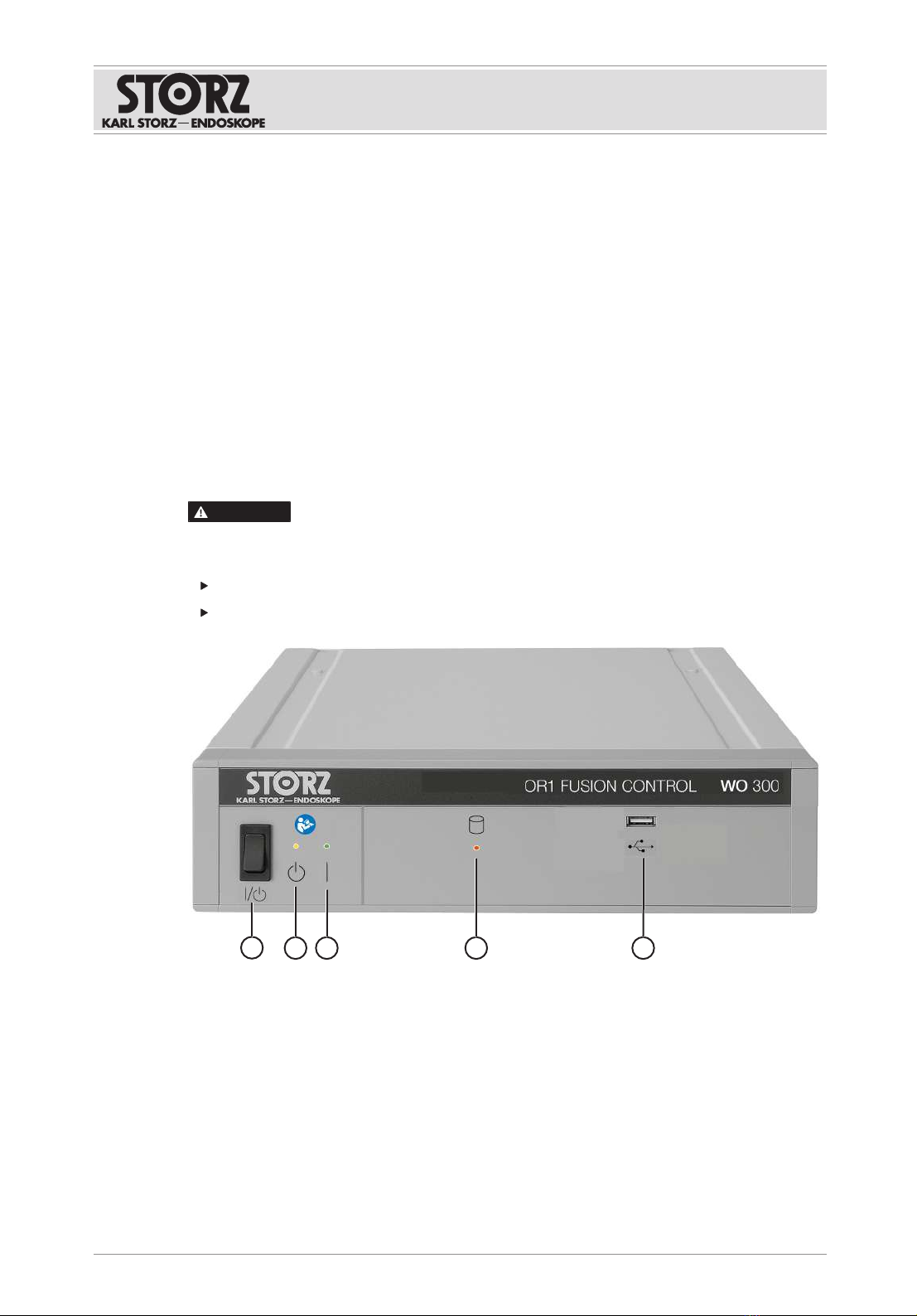
Symbol Meaning
Manufacturer
Date of manufacture
Medical device
Article no.
Serial number
Number of products in the product packaging
Unique Device Identifier
Consult the printed or electronic instructions for use
Fragile, handle with care
Keep dry
Humidity limit

Product description
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 15
Symbol Meaning
Air pressure limit
Federal (USA) law restricts this device to sale by or on the order of a
physician.
CE marking
With this mark, the manufacturer declares the compliance of the devices
with the applicable standards and directives
The device must not be modified in any way
4.4.2 Symbols on the product
Symbol Meaning
Follow instructions for use
‘Standby’
When the yellow indicator light is on, the device is in standby mode.
‘ON’
When the green indicator light is on, the device is switched on.
‘Access to hard disk’
When the red indicator light is on, the hard disk is being accessed.
USB port
Dangerous voltage
Danger of an electric shock!
The potential equalization is responsible for equalizing the potentials of
different metal parts that can be touched at the same time, or for reduc-
ing potential differences that could occur between the body, electromed-
ical devices, and external live parts during use.

Product description
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 16
4.4.3 Symbols on the type plate
Symbol Meaning
Manufacturer
CE conformity mark
With this mark, the manufacturer declares the compliance of the prod-
ucts with the applicable regulation (EU) 2017/745. A code number after
the CE mark indicates the responsible notified body.
Medical device
Federal (USA) law restricts this device to sale by or on the order of a
physician.
CSA certification mark for the USA and Canada
With this mark, the manufacturer declares compliance with the certifica-
tion requirements.
Environmental protection use period of 10 years (China RoHS)
Alternating current
Date of manufacture
Separate collection of electrical and electronic devices.
Do not dispose of in household refuse.
Serial number
4.5 Ambient conditions
Storage/transport conditions
Temperature -10°C...+60°C
Relative humidity
(non-condensing)
5–95%
Air pressure 500–1,080hPa
Operating conditions
Temperature 5°C...35°C
Relative humidity
(non-condensing)
20–80%
Air pressure 700–1,080hPa
Max. operating altitude 3,000m
Preparation
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 17
5 Preparation
5.1 Unpacking the product
1. Carefully remove the product and accessories from the packaging.
2. Check the delivery for missing items and evidence of shipping damage.
3. In the case of damage, hidden defects, and short deliveries, document their nature and
extent and contact the manufacturer or supplier immediately.
4. Keep packaging for further transport.
5.2 Setting up the device
The product can be operated free-standing, in a rack, or as a wall-mounted solution in either a
vertical or horizontal position.
1. Clean and disinfect the product thoroughly before using for the first time.
2. Observe the ambient conditions, see section Ambient conditions [p.16].
3. Observe the technical data, see section Technical data [p.13].
4. Install the product out of reach of patients.
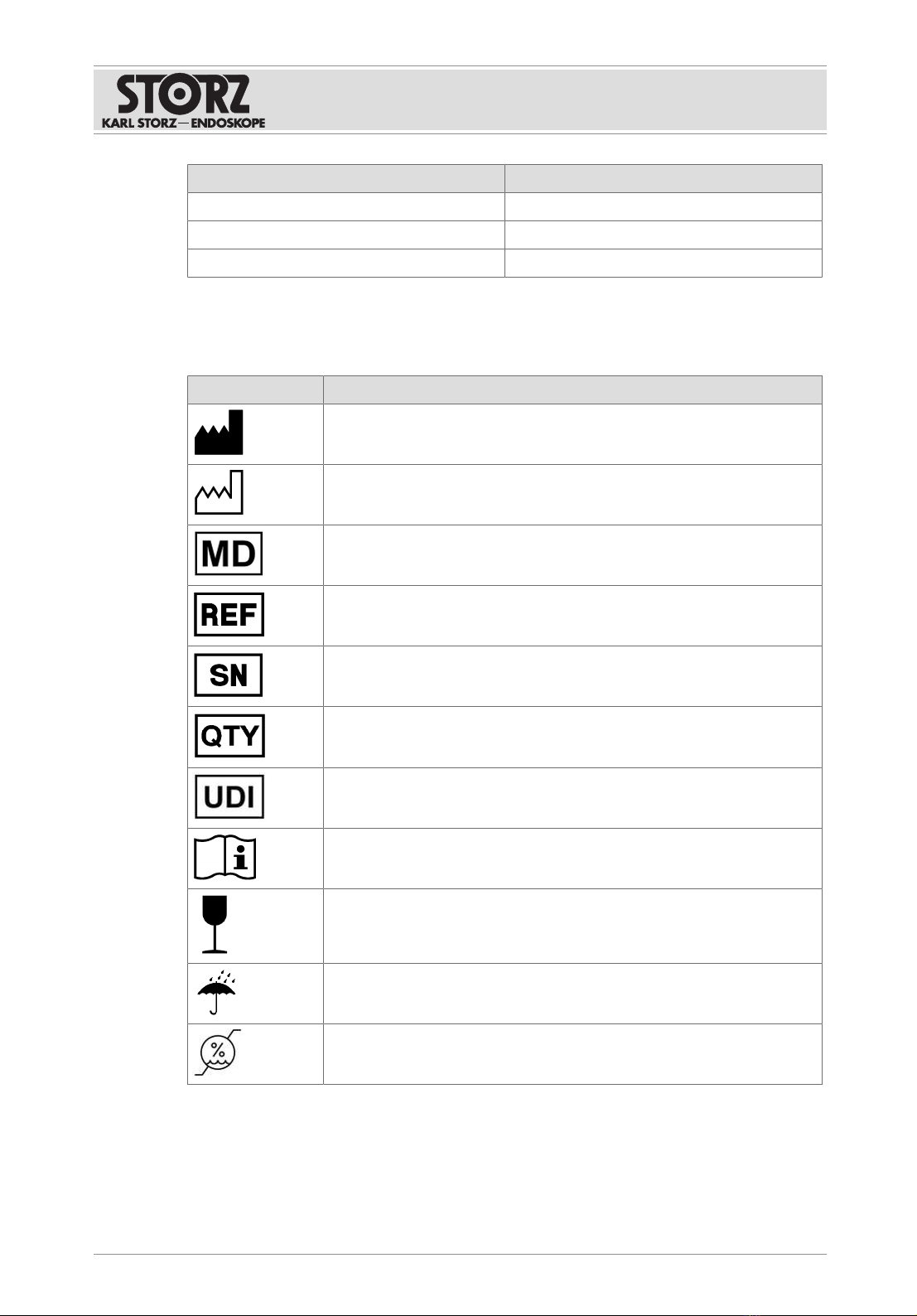
5. Note the free area dimensions based on the graphic to guarantee that the product is
sufficiently ventilated.
85,9 85,9310
20
375
20
4
1 2 3
5
1 Air inlet (max. temperature 35°C) 4 Front
2 Housing 5 Defined free areas
3 Air outlet
Nothing may be mounted in the defined free areas.
5.3 Connecting the product
The product may only be connected to other components by service personnel or personnel
authorized by .
Operation
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 18
6 Operation
6.1 Switching on the product
1. Switch on the connected equipment.
2. Switch on the product via the rocker switch.
ðThe software will start up.
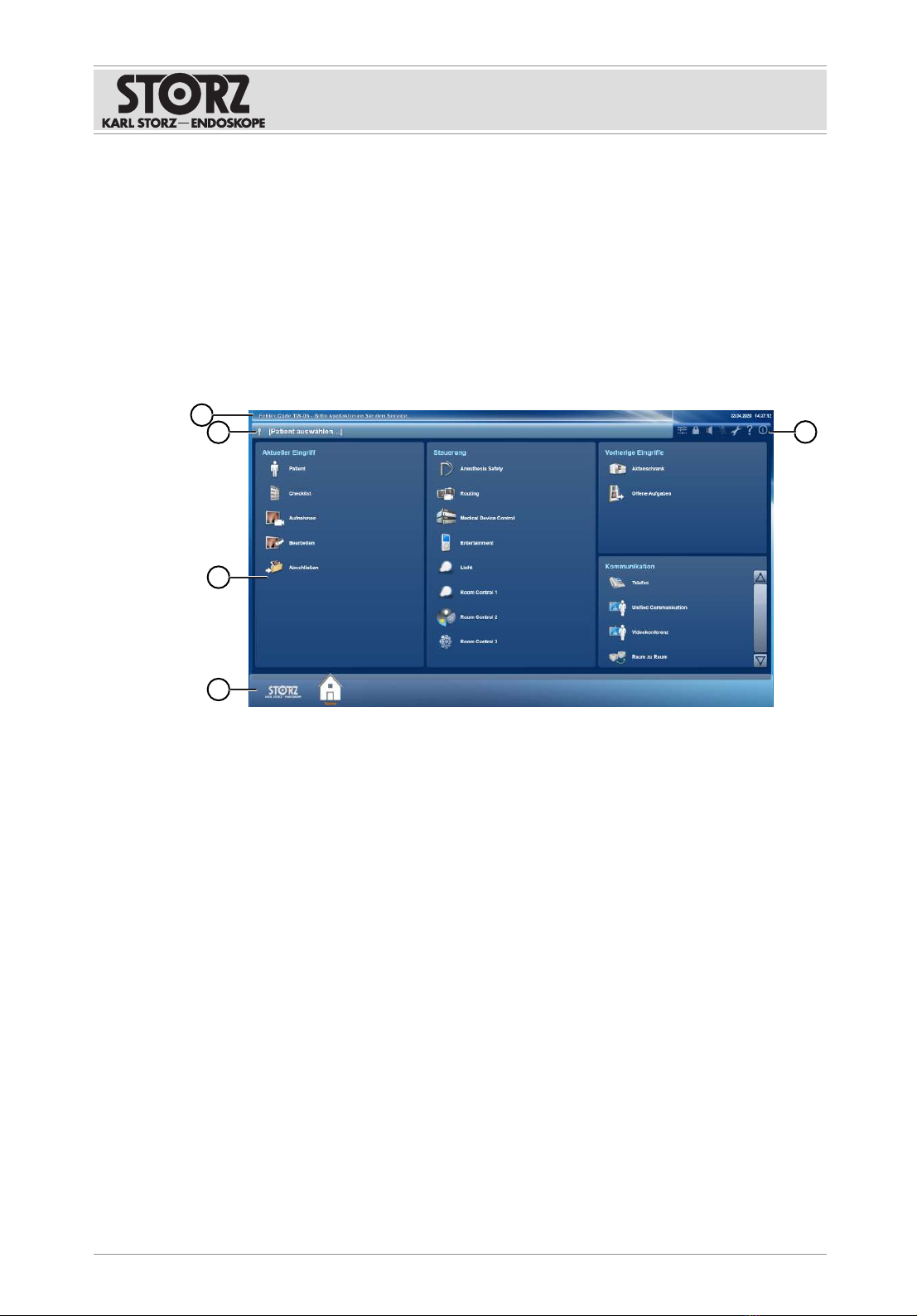
6.2 User interface
The following user interface appears when the is started:
4
1
2
3
5
1 Messages 4 Navigation bar
2 Patient data 5 Functions
3 Application areas
Messages
System information and notes appear in the top bar.
Patient data
The following data for the current patient appears if patient data is entered:
– Gender
– Last name
– First name
– Date of birth
– Age
– Patient ID
Application areas
The application areas contain modules that can vary depending on the configuration:

Operation
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 19
Application area Modules
Current procedure Manage and edit current patient data; document procedures.
Control Monitor and control general system functions that are not di-
rectly linked to upcoming or ongoing procedures.
Previous procedure Display patient documentation and complete the documentation
of previous procedures.
Communication Optional communication channels
Functions
System functions can be called up via the symbols, see section Symbols on the user interface
[p.19].
Navigation bar
The modules appear in the navigation bar when an application area is selected:
123
1 Main menu 3 Modules
2 Display
The following appears in the display:
– Incoming phone calls
– Incoming Unified Communication
– Request for video conference
– Request for a room-to-room connection
6.2.1 Symbols on the user interface
Symbol Meaning
Presets
Assign and save settings from the Capture, Routing, and Light modules to pro-
cedures and/or physicians.
Access status
Public, private, room to room. Private is set as the default.
Microphone
Switch microphone to mute. The microphone is set to mute as standard and
must be activated manually.
Volume
Adjust the output level of the active speakers or headphones.
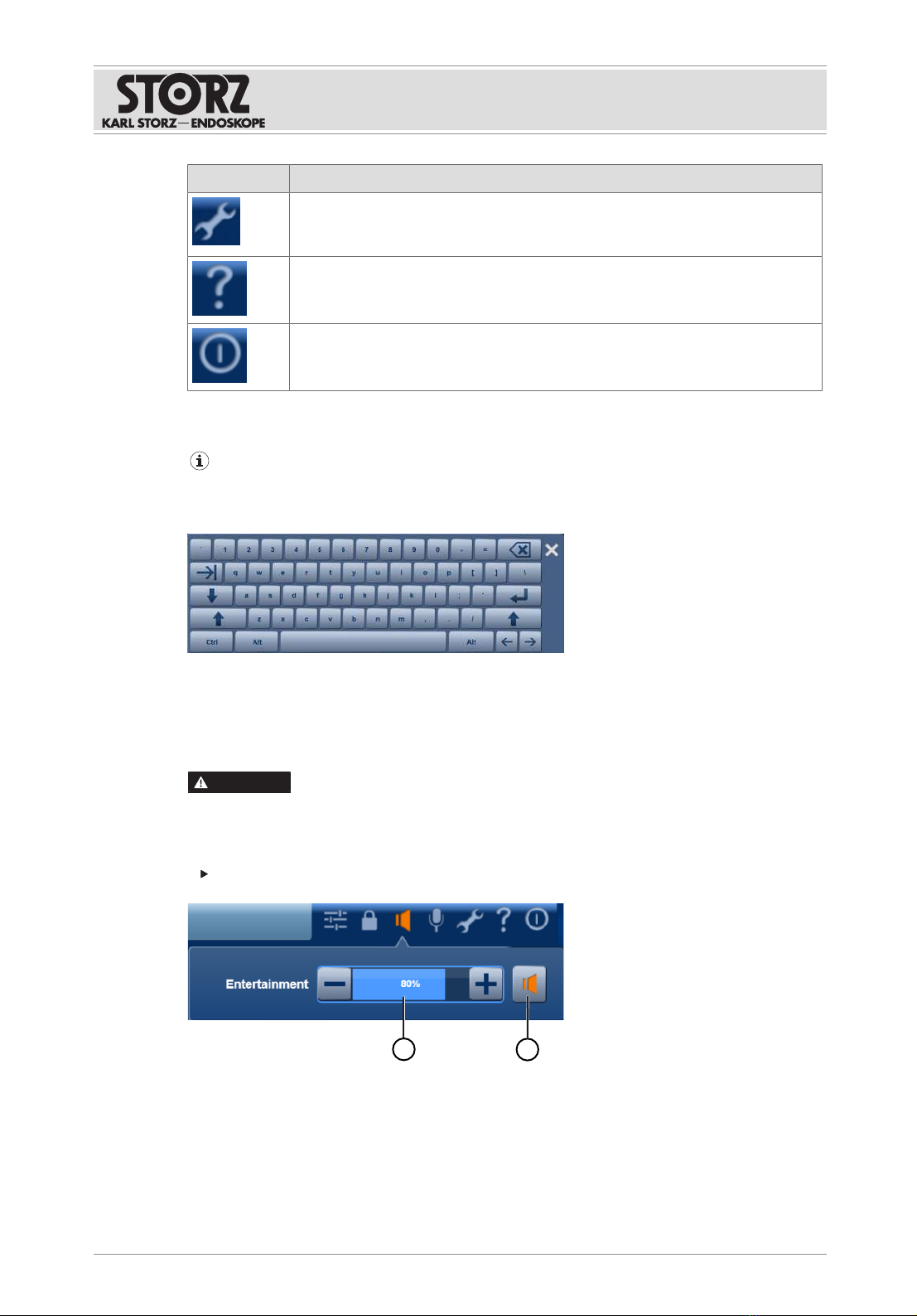
Operation
Instructions for use • KARL STORZ OR1 FUSION®, Rel. 1.4.2 • JEB924_EN_V1.0_02-2021_IFU_CE-MDR 20
Symbol Meaning
Settings
Select the functional scope and application-specific settings. This area is
password-protected.
System information
View product data and license information and save it to a USB stick.
Exit
Exit , shut down OR1 FUSION CONTROL®, or log out the user.
6.3 Onscreen keyboard
The onscreen keyboard may not available, depending on the configuration.
The onscreen keyboard is the same as a standard PC keyboard and is used in exactly the
same way.
The onscreen keyboard is only active when fields are being edited.
6.4 Volume control
Settings for speakers
WARNING
High volume! Risk of injury!
A high volume could startle the surgeon. This can result in injury to the patient, surgeon, or
third parties.
Check the volume before each operation.
12
1 Volume level
2 Speaker ON/OFF
Table of contents
Other Storz Analytical Instrument manuals
Storz
Storz 11301 BNX Series User manual

Storz
Storz C-MAC S User manual

Storz
Storz 11302 BDX User manual

Storz
Storz OR1 .avm User manual

Storz
Storz VIDEOSCOPE User manual

Storz
Storz 11272 VN User manual

Storz
Storz 112 Series User manual
Storz
Storz 11272 VA User manual

Storz
Storz C-MAC 8401 Series User manual
Popular Analytical Instrument manuals by other brands

Perkins
Perkins ROVAC 9671A owner's manual

Teledyne
Teledyne 327RACEU instruction manual

Virax
Virax Mini VISIOVAL user manual
Teledyne Analytical Instruments
Teledyne Analytical Instruments 3020 M operating instructions

Spartan Tool
Spartan Tool Explorer M130 product manual

SW-Stahl
SW-Stahl 32295L instruction manual











