Terumo Harvest SmartPReP 2 Operating manual

4531200 / 1042
Operators and Service Manual
SmartPReP® 2 Centrifuge System
For Platelet, Bone Marrow
And Adipose Tissue Concentration

Page 2of 26 4531200 / 1052
SmartPReP®2 SYSTEM
OPERATOR’S AND SERVICE MANUAL
Table of Contents
ABOUT THIS MANUAL...................................................................................................................4
INTENDED USE ..............................................................................................................................4
SYSTEM CONCEPT .........................................................................................................................4
DESCRIPTION (SMP2 with serial numbers 0101-5999).................................................................5
Hardware..................................................................................................................................5
DESCRIPTION (SMP2 with serial numbers 6000 and greater) ......................................................5
Hardware..................................................................................................................................5
ACCESSORIES.................................................................................................................................7
HARDWARE SET-UP.......................................................................................................................8
INSTRUCTIONS FOR USE ...............................................................................................................8
Loading the Process Disposables –APC & BMAC.....................................................................9
Loading the Process Disposables –AdiPrep Adipose Transfer System................................. 10
CONTRAINDICATIONS ................................................................................................................ 10
WARNINGS................................................................................................................................. 10
CAUTIONS .................................................................................................................................. 11
SAFETY........................................................................................................................................ 12
SYMBOLS.................................................................................................................................... 12
TROUBLESHOOTING CHART....................................................................................................... 13
MAINTENANCE........................................................................................................................... 15
SERVICE ...................................................................................................................................... 16
WARRANTY SERVICE.............................................................................................................. 16
NON-WARRANTY WORK........................................................................................................ 16
RETURNED GOODS POLICY ........................................................................................................ 17
AUTHORIZATION ................................................................................................................... 17
REQUEST FOR RETURN AUTHORIZATION.............................................................................. 17
FREIGHT (RETURNED GOODS)............................................................................................... 17
LIMITED WARRANTY .................................................................................................................. 17
TECHNICAL INFORMATION (SMP2 serial numbers 0101-5999) ................................................ 18
PHYSICAL SPECIFICATIONS .................................................................................................... 18

Page 3of 26 4531200 / 1052
STANDARDS ........................................................................................................................... 18
ELECTRICAL SPECIFICATIONS................................................................................................. 19
ENVIRONMENTAL CONSIDERATIONS.................................................................................... 19
ELECTROMAGNETIC COMPLIANCE……………………………………………………………………………………19
TECHNICAL INFORMATION (SMP2 serial numbers 6000 and greater)...................................... 22
PHYSICAL SPECIFICATIONS .................................................................................................... 22
STANDARDS ........................................................................................................................... 22
ELECTRICAL SPECIFICATIONS................................................................................................. 23
ENVIRONMENTAL CONSIDERATIONS.................................................................................... 23
ELECTROMAGNETIC COMPLIANCE…………………………………………………………………………………….23
Page 4of 26 4531200 / 1052
ABOUT THIS MANUAL
Messages that are headed by “NOTE:” indicate information or procedures that if not followed correctly
can cause improper results.
Messages that are headed by “CAUTION:” indicate information or procedures that if not followed correctly
can cause improper results and damage to the equipment.
Messages that are headed by “WARNING:” indicate information or procedures that if not followed
correctly can cause improper results, damage to the equipment, injury to personnel, or serious patient
harm.
INTENDED USE
The SmartPReP 2 System is designed to be used for the safe and rapid preparation of autologous
platelet-rich-plasma (PRP) from a small sample of blood at the patient’s point of care. The PRP can be
mixed with autograft or allograft bone grafting materials prior to application to an orthopedic surgical
site as deemed necessary by the clinical use requirements.
The SmartPReP2 System is intended to be used in the clinical laboratory or intraoperatively at point-of-
care for the safe and rapid preparation of platelet poor plasma and platelet concentrate from a small
sample of blood and for preparation of a cell concentrate from bone marrow.
The AdiPrep™ Adipose Transfer System is used in medical procedures involving the harvesting and
transplanting of autologous tissue. The AdiPrep system is used for concentrating adipose tissue
harvested with a legally marketed lipoplasty system. The AdiPrep™ Adipose Transfer System is
intended for use in the following surgical specialties when the concentration of harvested adipose
tissue is desired: Arthroscopic Surgery, Gastrointestinal Surgery, General surgery, Gynecological
Surgery, Laparoscopic Surgery, Neurosurgery, Plastic and Reconstructive Surgery, Thoracic Surgery, and
Urological Surgery.
SYSTEM CONCEPT
The SmartPReP 2 System consists of a microprocessor-controlled, centrifuge with automated decanting
capability using a dual chamber sterile processing disposable. The system technology provides a
reproducible process for concentrating desired cells without user interaction beyond loading and starting
the centrifuge.

Page 5of 26 4531200 / 1052
DESCRIPTION (SMP2 with serial numbers 0101-5999)
Hardware
The SmartPReP 2 System (SN 0101-5999) is similar to a general purpose
swinging bucket centrifuge with the following exceptions:
The bucket insert accepts only the Harvest processing disposable
(PD)
The bucket orientation (horizontal or vertical) is automatically
controlled in order to perform decanting
The automated dual-spin centrifugation process is fixed and cannot
be modified by the user, thus ensuring reproducible results
The control panel is simple and intuitive, providing only three
controls: LID, START and STOP
The (LCD) liquid crystal display indicates the time remaining until the process is complete.
Control Panel
POWER Illuminates when the unit is plugged in.
INTERRUPTED Upon initial power up the INTERRUPTED LED will illuminate, this is normal operation.
It also illuminates when the control system senses an electronic failure. If this
condition is detected, the centrifuge is brought to a stop and cannot be restarted until
the fault condition is corrected. The INTERRUPTED LED is also illuminated whenever
the STOP keypad is pressed during a process cycle.
LID OPEN Illuminates when LID is open or improperly closed. When this LED is illuminated, the
centrifuge is prevented from operating.
IMBALANCE Illuminates when the control system senses an imbalance in the rotor. If this condition
occurs, the centrifuge is brought to a stop and cannot be restarted until the imbalance
condition is corrected.
TIMING Displays the time remaining in the process cycle.

Page 6of 26 4531200 / 1052
DESCRIPTION (SMP2 with serial numbers 6000 and greater)
Hardware
The SmartPReP 2 System (SN 6000 and greater) is similar to a general purpose
swinging bucket centrifuge with the following exceptions:
The bucket insert accepts only the Harvest processing disposable (PD)
The bucket orientation (horizontal or vertical) is automatically
controlled in order to perform decanting
The automated dual-spin centrifugation process is fixed and cannot be
modified by the user, thus ensuring reproducible results
The control panel is simple and intuitive, providing only four controls:
LID, PRP (START), BMAC (START) and STOP.
The (LCD) liquid crystal display indicates the time remaining until the process is complete.
POWER
INTERRUPTED
LID OPEN
IMBALANCE
DONE
~
TIMING
LID
STOP
PRP BMAC
0
Control Panel
POWER Illuminates when the unit is plugged in.
INTERRUPTED Upon initial power up the INTERRUPTED LED will illuminate, this is normal operation.
It also illuminates when the control system senses an electronic failure. If this
condition is detected, the centrifuge is brought to a stop and cannot be restarted until
the fault condition is corrected. The INTERRUPTED LED is also illuminated whenever
the STOP keypad is pressed during a process cycle.
LID OPEN Illuminates when LID is open or improperly closed. When this LED is illuminated, the
centrifuge is prevented from operating.
IMBALANCE Illuminates when the control system senses an imbalance in the rotor. If this condition
occurs, the centrifuge is brought to a stop and cannot be restarted until the imbalance
condition is corrected.
TIMING Displays the time remaining in the process cycle.

Page 7of 26 4531200 / 1052
SMP2 Control Panels
Serial Number 0101- 5999
Serial Number 6000 >
Open Lid
Process
Platelet Concentrate
Process
Adipose Concentrate
Process
Bone Marrow Concentrate
Stop System
ACCESSORIES
The SmartPReP 2 System is compatible with Autologous Platelet Concentrate (APC+) Procedure Packs,
Bone Marrow Aspirate Concentrate (BMAC) Procedure Packs, and AdiPrep Adipose Transfer System
(AATS). Please refer to the Instructions for Use provided with each of the kits listed below for proper use
of those kits.*HAT kits not available in the U.S.
Balance Weight Chart
BW-20 Balance Weight
TA-20 Balance Weight/
Tube Adapter
Used with HAT Kit*. BW-20 is
needed for opposing rotor
BW-60 Balance Weight
TA-60 Balance Weight/
Tube Adapter
Used with HAT Kit*. BW-60 is
needed for opposing rotor
BW-30 Balance Weight
TA-30 Balance Weight/
Tube Adapter
Used with HAT Kit*. BW-30 is
needed for opposing rotor
AA-1 AdiPrep Adapter
Used with AATS Kits
2- AA-1 are required to process
BW-ADI AdiPrep Balance Weight
Used with AA-1 Adapter and AATS Kits
APC-120 & BMAC-120 kits do not require balance weights
BWAT-20 & BWAT-60 balance weights are avialable for international use with AT Pro Kits
Page 8of 26 4531200 / 1052
HARDWARE SET-UP
The SmartPReP 2 System is packaged in one box. Verify that the box contains one (1) SmartPReP 2
centrifuge, line cord, and Operator’s and Service Manual.
NOTE: Save box, packaging and rotor support foam for future use.
1. Remove the SmartPReP 2 centrifuge from the box and place on a sturdy counter or stable cart.
Remove all shipping restraints and inspect for damage.
2. Install line cord and plug into the appropriate mains voltage.
3. Visually inspect the display panel and verify the Green “Power” light and the yellow “Interrupted”
light are illuminated. Press the LID keypad and open the lid. The “Interrupted” light should go out
and the yellow “Lid Open” light should be illuminated.
4. Remove all shipping material. Verify that the rotor assembly is correctly installed and secure on the
motor shaft.
5. Remove the balance weights from the rotor assembly and set aside.
6. Commence a test run by closing the lid firmly; verify that only the POWER light is illuminated; press
START, PRP or BMAC. The centrifuge should start and the automated cycle should be complete in
approximately 14 minutes. At the end of the cycle, the “DONE” indicator light should be illuminated
and audible tone sounds, indicating the rotor has come to a complete stop and it is safe to open
the centrifuge cover. The centrifuge is now ready to process blood, marrow or adipose tissue.
NOTE: Follow established protocols and procedures prior to placing the SmartPReP 2 System into service.
NOTE: If additional protective earthing (grounding) is required use the green/yellow terminal on the back
of the instrument.
CAUTION: Do not force processing disposable into rotor trunnion (swinging buckets). The processing
disposable(s) should fit snuggly but should not require excessive force to install. If resistance is
experienced, check for obstructions in the rotor and/or debris on the process disposable or
proper orientation of the process disposable to the swinging bucket.
INSTRUCTIONS FOR USE
Once the hardware is set up and has successfully completed a test run the unit is ready to operate. Any
one of the following five scenarios will automatically balance the unit if the Process Disposables (PD) are
filled properly. (Refer to the kit’s Instructions for Use for detailed filling instructions). The standard
centrifuge is equipped with a two place rotor. An optional four place rotor is available through special
order.
CAUTION:Do not process a combination of 20, 30 and 60mL APC or BMAC PD’s in the same cycle as they
will not balance.
CAUTION:Do not process APC or BMAC PD’s with the AdiPrep PD in the same cycle as they will not
balance.
CAUTION: Always have another PD or BW of equal weight in the trunnion opposing the PD to ensure
balance.

Page 9of 26 4531200 / 1052
Two Place Rotor
Scenario 1 –One properly filled equal volume PD in each trunnion. (2 total)
Scenario 2 –One properly filled PD in one trunnion and one balance weight (of equal weight) in the
opposing trunnion.
Four Place Rotor
Scenario 3 –Same as above for one or two PD’s
Scenario 4 –One properly filled equal volume PD in each swinging trunnion. (4 total)
Scenario 5 –One properly filled PD in three of the trunnion and one balance weight (of equal
weight) in the fourth trunnion bucket. (3 total)
CAUTION: If the unit is not loaded with one of the five scenarios listed above the unit will IMBALANCE
and not continue.
CAUTION: The process disposables are single patient use only. Do not re-use or re-sterilize. Reuse of
disposable products may cause illness, injury or even death. Discard all unused components at
the end of the procedure.
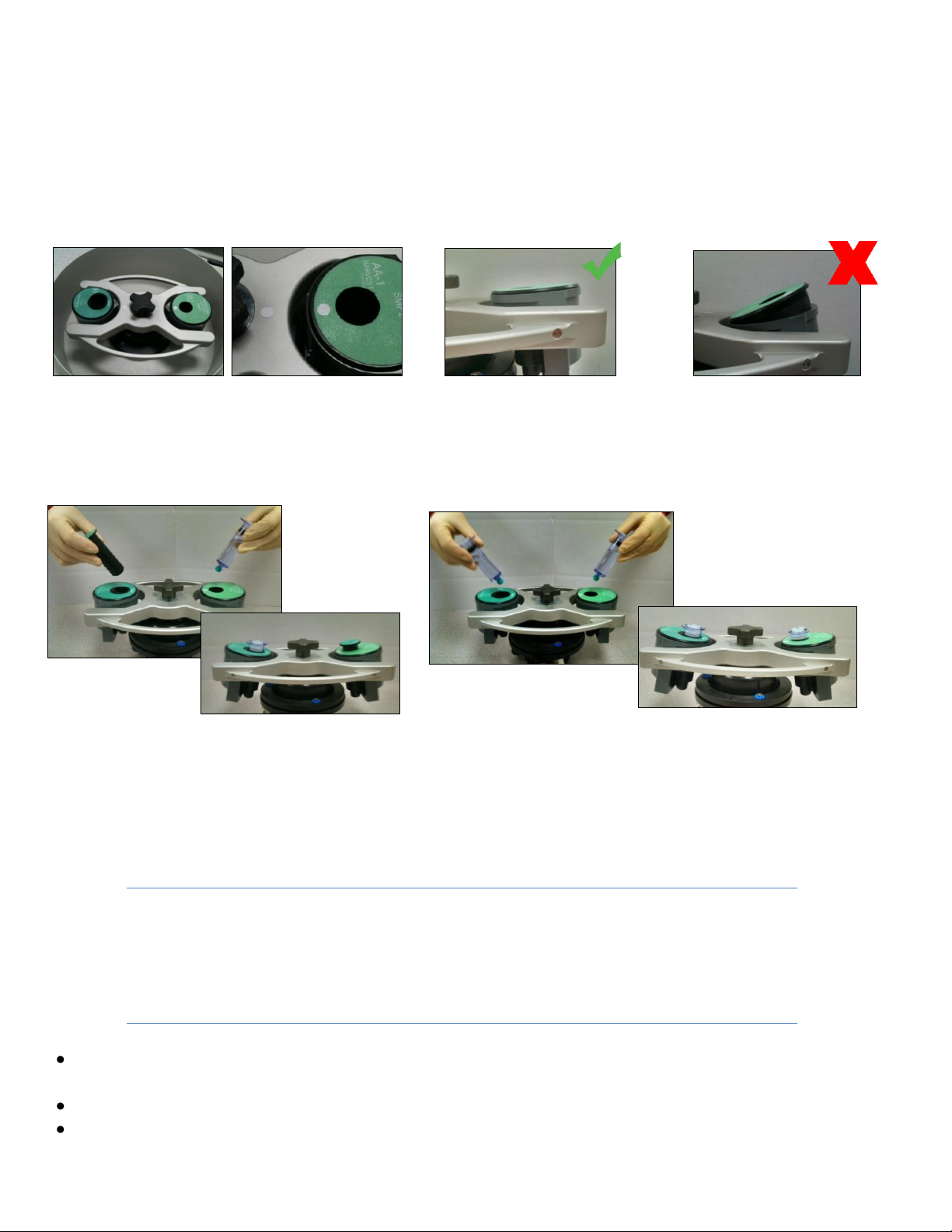
Loading the Process Disposables –APC & BMAC
1. Press the LID button and open the lid
2. Load the swinging trunnions as described in one of the five scenarios. Be sure to line up the “white
dot” indicator on the trunnion with the white dot on the PD. Rotate the PD as necessary to ensure
proper alignment.
CAUTION: Be sure the PD is properly seated as shown
3. Close the lid
4. Press the START button in SMP2 with serial numbers 0101-5999: For SMP2 Systems with serial
numbers 6000> press the blue PRP button for platelet concentrate or orange BMAC button for
bone marrow concentrate.
5. The TIMING window will display the remaining time till the end of the cycle. When the cycle is
complete the DONE led will flash and an audible alarm will sound.
6. Press the LID button and remove the PDs.
7. Refer to the Instructions for Use accompanying the Processing Kits.
Page 10 of 26 4531200 / 1052
Loading the Process Disposable –AdiPrep Adipose Transfer System
Note: The Adipose Hardware Kit (AHK-1) is required to operate the SMP2 System with the AdiPrep process disposable.
See Scenarios 1 and 2 for adipose processing.
Install the two adapters into the rotor trunnions
with the white dots aligned as shown.
Be sure the adapter is properly seated
as shown below
Correct Incorrect
To process adipose tissue in the SMP2 System:
Scenario 1 - Install one AdiPrep process disposable
into one AA-1 Adapter and the BW-ADI AdiPrep
Balance Weight into the opposing adapter, as
shown below.
Scenario 2 - Install two (2) AdiPrep process disposables, one in
each AA-1 Adapter, as shown below.
1. Close the lid.
2. Press the green START or the blue PRP button.
3. The TIMING window will display the remaining time of the cycle. When the display indicates 10 (approximately 4
minutes have passed) press the red STOP button.
4. When the rotor has stopped, press the LID button and remove the PDs.
CONTRAINDICATIONS
The use of the SmartPReP 2 System may be contraindicated when there is: Clinical or laboratory evidence
of septicemia for patients who have taken aspirin, or other medications that alter platelet function, within
3 days prior to surgery, or patients with disorders associated with platelet dysfunction.
WARNINGS
Federal law (USA) restricts this device to sale by, or on the order of a physician. The physician is solely
responsible for the use of this device.
Safe and effective use of this device requires proper set-up and operation by trained personnel.
This device should not be used in the presence of flammable agents.

Page 11 of 26 4531200 / 1052
Risk of electrical shock, do not attempt to service electrical components; refer servicing to qualified
personnel.
Plasma, platelets and cell concentrate prepared with this system are not intended for transfusion.
Changes or modifications to this unit not expressly approved by the party responsible for compliance
could void the user's authority to operate the equipment.
BMAC Processing: The safety and effectiveness of this device for in-vivo indications for use has not
been established.
CAUTIONS
Read all instructions prior to use.
Health professionals responsible for blood collection must be trained in the practice of venipuncture
and be aware of the inherent risks. Aseptic technique, proper skin preparation and continued
protection of the venipuncture site are essential.
Bone marrow aspirate should be collected by or under the supervision of a qualified physician trained
in this procedure.
All blood and marrow components should be handled as if infectious. To minimize the potential for
exposure to blood-borne pathogens observe UNIVERSAL PRECAUTIONS when handling blood and
blood components.
Disposables that have come in contact with blood, bone marrow, or adipose tissue are to be
considered hazardous waste. Follow established hospital protocols and procedures for handling
hazardous waste including but not limited to: discard in appropriate, leak-proof container marked with
a biohazard symbol, double bag or place hazardous waste in a protective container, discard sharps in
rigid, puncture-proof and leak-proof “sharps” container. Incineration and decontamination by
autoclaving are the currently recommended methods for disposing of blood/marrow/adipose tissue
samples and blood /marrow/adipose tissue products.
Aseptic technique should be used when aspirating and dispensing fluids.
Commercially available solutions (USP, NF) or pharmacy prepared must be sterile.
Heparin-induced thrombocytopenia may occur in approximately 5% patients receiving heparin therapy.
This condition may cause hypercoagulability and may interfere with process results.
Actual performance may vary depending on operating conditions. Check sample at end of process to
confirm that fluids are properly separated. If the separation system fails for any reason, the
blood/marrow/adipose tissue should not be recovered or reprocessed by any other method.
Blood/marrow/adipose tissue should be disposed of in accordance with policies consistent with
disposal of biohazardous waste.
Report needle sticks immediately and follow established protocol. Percutaneous puncture with a
contaminated needle may lead to serious illness such as hepatitis, HIV (AIDS) or other infectious
disease. Resheathing needles is dangerous.
Do not use if the packaging is open or damaged.
DO NOT RESTERILIZE.
Disposable is single patient use only. Discard all unused components at the end of the procedure.
Re-use may lead to infection or illness/injury/death.
Separated blood/marrow products should be used within four (4) hours of collection.
Page 12 of 26 4531200 / 1052
SAFETY
Safety features for the SmartPReP 2 System are constantly monitored and controlled to ensure safe
operation of the unit. The possible system faults that are monitored are:
Open Lid: A sensor on the lid ensures that the SmartPReP 2 system is never operated with the lid
open.
Out of Balance: An out of balance detector has been incorporated into the SmartPReP 2 system to
ensure that imbalances in the rotor are not allowed to create an unsafe operating condition.
Stop Button: A stop button is provided on the User Interface Panel to allow the user to stop the
process safely and quickly.
SYMBOLS
If applicable, the following symbols may appear on the SmartPReP 2 System:
Attention, Consult
Accompanying Documents
Power on
Start
WEEE Compliant Producer
Stop
Do Not Reuse
Open Lid
STERILE R EO A
Sterilized Using Irradiation,
Ethylene Oxide, Steam
or Dry Heat
Equipotential
LOT
Lot Code
Alternating Current
Manufacturer
Interrupted
Consult Instructions for Use
Imbalance
Do not use if Package is
Damaged
Hazardous Voltage
Use By
Do Not Use in the Presence of
Flammable Anesthetics
EC REP
Authorized Representative in the
European Community
Timing (Remaining cycle time)
R Only
x
Caution: Federal law restricts this
device to sale by or on the order
of a physician
Process has completed
R
Catalogue Number

Page 13 of 26 4531200 / 1052
TROUBLESHOOTING CHART
Symptoms
Possible Cause
Possible Solution
Cannot resolve
means go to next step
Interrupted
Interrupted light is on.
Customer hasn’t opened
the lid yet
Open the lid and it will clear the interrupt
circuit. This is designed to happen on first
power up!
Interrupted light is on.
The Stop button was
accidentally pushed.
Press the Lid Open button, close the lid and
retry.
Interrupted light is on.
The display label is
contacting the Stop
button.
Cycle the Stop button then retry.
Interrupted light is on.
There was a power drop
(brown out).
Press the Lid Open button, close the lid and
retry.
Interrupted light is on.
The Printed Circuit has
failed. Interference.
Cycle the power a few times. If an emergency
call Harvest Tech Service at 877-842-7837
Contact Harvest
Lid Open
Lid Open light is on
The lid is not completely
closed
Open the lid and then close it again. Push
down on the lid.
Lid Open light is on
Safety circuit is missing
Check for magnet assembly. Open lid and look
to the right of the latch assembly for a small
plate with a round magnet attached inside the
lid lip.
Contact Harvest.
Imbalance
Imbalance light is on
Incorrect balance weight
used
Confirm that your process disposable volume
and the balance weight are correct.
Imbalance light is on
The machine was
damaged in shipping
Power cycle the machine and retry.
Imbalance light is on
The machine was bumped
after start
Power cycle the machine and retry.
Contact Harvest.
Lid won’t open
Lid won’t open
The customer hasn’t
waited for the rotor to
stop spinning
Lid will not open while processing and/or
rotor is spinning.
Lid won’t open
Lid solenoid has failed.
Machine must be returned
Contact Harvest for
RGA#.
No decant
No decant
Decant solenoid(s) have
failed
Verify there is no decant at all. You can
manually decant by tipping the PD, and spin
the PD again to finish the process
No decant
Printed Circuit Board has
failed.
Machine must be returned for repair. You can
manually decant by tipping the PD, and spin
the PD again to finish the process
Contact Harvest for
RGA#.
No decant
Stuck shelf and/or clotting
Verify the shelf location is not on bottom.
Was there separation?
Contact Harvest for
RGA#.
No decant
Clotting
Repeat –ensure proper ACD mixing during
draw. Poor mixing causes clotting.
Test machine, run
empty to verify decant
function.

Page 14 of 26 4531200 / 1052
Symptoms
Possible Cause
Possible Solution
Cannot resolve
means go to next step
No power
No power
Not plugged in
Verify that the machine is plugged into
appropriate voltage 100V/115V/230V. Try
powering another device. Check wall switch,
power strip?
No power
Fuses are blown.
Check the fuses. Fuses must be replaced by
approved service technician.
Contact Harvest for
RGA#.
No Platelet Concentrate
No platelet concentrate
See ‘No decant’ section
also
Verify the machine is decanting. Restart
process and verify decant.
No platelet concentrate
Clotting
Repeat process with a new PD –ensure
proper ACD mixing during draw. Poor mixing
causes clotting.
Test the SMP2
machine, run empty to
verify decant function.
No platelet concentrate
High RBC count
Verify patient’s blood count
Contact Harvest.
Too loud
Too loud
Rotor is loose
Fully seat and tighten the rotor knob
clockwise.
Too loud
Disposables are not seated
correctly.
Verify that the disposables are fully seated
PD’s should not touch lid glass.
Test the SMP2
machine, run empty to
verify decant function.
Too loud
Disposable vs. BW.
Weights are on the
imbalance threshold.
Run the machine empty to verify noise level. If
it is quiet empty, then blood volume is not
balanced.
Verify weights.
Too loud
Loose hardware or screws
near the feet.
Check all hardware. Try to isolate location.
Contact Harvest for
RGA#.
Too loud
Missing rubber feet
Check that all 4 feet are intact, Harvest can
provide replacement feet.
Contact Harvest
Shipping damage
Shipping damage
Shipping damage, dents
etc.
Replacement machine may be sent
Contact Harvest for
RGA#.
Contact Harvest Technical Service Toll Free # 1-877-842-7837
Page 15 of 26 4531200 / 1052
MAINTENANCE
The SmartPReP 2 System requires no calibration and is designed for minimum maintenance. Internal
software monitors every process run from start to finish. If the timing or RPM vary more than the
specifications listed below the unit will stop and the Interrupted light will illuminate.
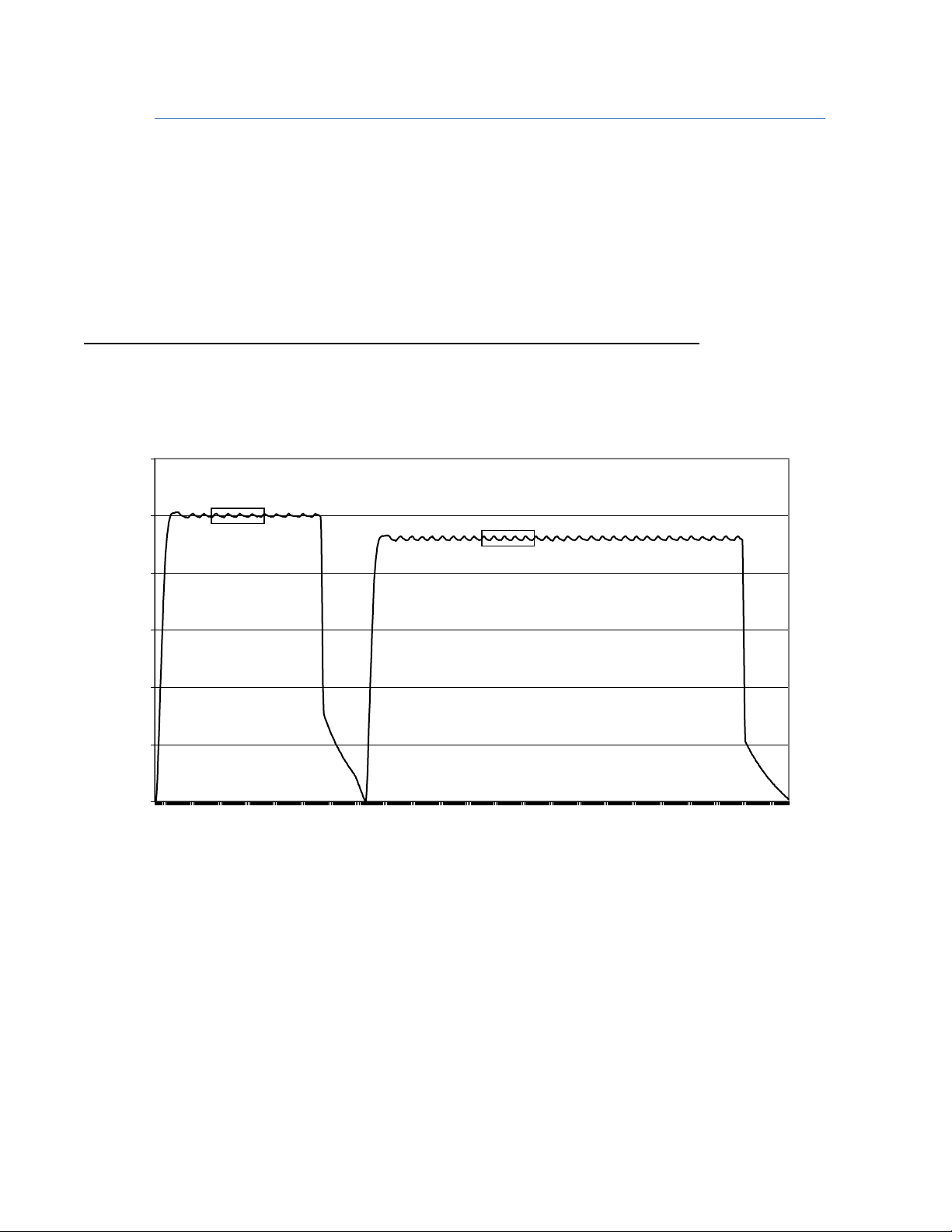
Optional - SPEED VERIFICATION
The centrifuge has a set speed protocol with two points that can be confirmed as follows:
Time Speed should be
A 1-3 minute(s) into process 2500RPM±150 (2350 - 2650)
B 6-9 minutes into process 2300RPM±140 (2160 - 2440)
The following lists the recommended maintenance procedures that should be followed.
CLEANING EVERY USE
CAUTION: Ensure line cord is disconnected from power source prior to cleaning. Do not use abrasive
cleaning agents. Do not use solvents or strong alcohol solutions. Do not immerse any part of the
SmartPReP 2 System in liquids.
The SmartPReP 2 System should be cleaned after each use and/or after any spill of liquids. For routine
cleaning use only mild detergents, water and a soft damp cloth to thoroughly clean the inside and outside
of the unit. Allow unit to dry before returning to service. A dry cloth or paper towels can be used to dry
the unit.
0
500
1000
1500
2000
2500
3000
1
36
71
106
141
176
211
246
281
316
351
386
421
456
491
526
561
596
631
666
701
736
771
806
RMP
Speed Check points
A B

Page 16 of 26 4531200 / 1052
DECONTAMINATION PROCEDURE AFTER BLOOD SPILLS or AS NEEDED
For disinfecting/decontamination, spray the unit with a 10% bleach/water solution and wipe with a damp
cloth that has been wet with the 10% bleach/water solution. Allow unit to dry before returning to service.
A dry cloth or paper towels may be used to dry the unit.
CAUTION: Ensure line cord is disconnected from power source prior to cleaning. Do not immerse any part
of the SmartPReP 2 System in liquid.
Before using any cleaning or decontamination methods except those recommended by the manufacturer,
users should check with the manufacturer that the proposed method will not damage the equipment.
GENERAL INSPECTION EVERY USE
Visually check the condition of the SmartPReP 2 System. Remove from service any unit that shows signs of
physical damage or one in which the PD does not easily install. Verify that device has all four rubber feet
secured.
ROTOR ASSEMBLY MAINTENANCE Monthly or AS NEEDED BASIS
Check movement of trunnions; there should be no binding or obstructions. Confirm the rotor nut is tight.
Hand tighten the nut, do not use a tool. Clean if necessary.
TEST RUN Monthly or AS NEEDED BASIS
Perform verification of centrifuge process from start to finish. Commence a test run by closing the lid
firmly; verify that only the POWER light is illuminated; press START, PRP or BMAC. Confirm the system
performs a first spin, coast down and stop, second spin, coast down and stop. Process is complete in
approx. 14 minutes.
SERVICE
Harvest Technologies, Corp., or its authorized agents must perform all service. Contact Harvest
Technologies at 508-732-7500 (Toll Free 1-877-8HARVEST in continental U.S.) or your local distributor or
sales representative.
USEFUL LIFE - WARRANTY SERVICE
The SmartPReP2 centrifuge has been validated to perform as intended for up to five (5) years from the
date of purchase. Units under warranty that are received for repair, that have not been obviously abused
or impact damaged, will be promptly repaired and returned at no charge. See the Limited Warranty
section of this manual. A no-charge purchase order is requested for tracking purposes.
NON-WARRANTY WORK
Units received that have suffered obvious abuse or impact damage and units no longer under warranty will
be promptly inspected and a verbal estimate of repair cost will be provided. A purchase order is required
from the hospital that is consistent with the verbal estimate. A written estimate for repair will be provided
upon request.

Page 17 of 26 4531200 / 1052
RETURNED GOODS POLICY
AUTHORIZATION
The customer must receive authorization from Harvest Technologies before merchandise can be returned.
Unauthorized returns will not be accepted and will be returned to the customer at the customer’s
expense.
REQUEST FOR RETURN AUTHORIZATION
The request for Return Goods Authorization Number (RGA) must include:
1. Serial number and/or lot number, catalog number, and quantity.
2. Reason for return.
3. Customer purchase order number and date.
4. Harvest Technologies invoice number and date.
5. Hospital or Doctors name, address and phone number.
CAUTION: The SmartPReP 2 System MUST BE CLEANED AND DISINFECTED PRIOR TO RETURN or it will be
immediately returned to sender as received. SmartPReP 2 System units returned for service
must have an intact Serial Number label. SmartPReP 2 Systems with missing or altered serial
numbers will be serviced as Non-Warranty repairs.
FREIGHT (RETURNED GOODS)
When authorized, all merchandise must be returned FREIGHT PREPAID. Any merchandise returned as
freight collect will be refused by Harvest Technologies and returned to the customer at the customer’s
expense.
LIMITED WARRANTY
The SmartPReP 2 System is warranted to be free from material and workmanship defects for a period of
one (1) year from the date of purchase; abuse and/or impact damage excluded. Harvest Technologies
reserves the right to replace any or all components in lieu of repair. Harvest Technologies will prepay
shipping costs, repair or replace the SmartPReP 2 System found to be defective during the warranty
period. This warranty does not cover misuse, impact damage, or obvious abuse of the device. No warranty
or affirmation of fact, expressed or implied, other than stated above, is made or authorized by Harvest
Technologies, and Harvest Technologies’ liability in all events is limited to the purchase price paid for the
device.
Ship to:
Include the following
Attn: Service Department
Returned Goods Authorization # (RGA)
Harvest Technologies, Corp.
40 Grissom Rd, Suite 100
Plymouth, MA 02360
Hospital Name
Address
Telephone Number
Contact Person
Description of the Problem
RGA #
PO Number

Page 18 of 26 4531200 / 1052
TECHNICAL INFORMATION
For SMP2 serial number 0101-5999
The Harvest SmartPReP 2 Centrifuge meets the requirements for leakage current and hi-pot testing listed
in BS EN 60601-1 and UL 2601-1 standards for Medical Electrical Equipment.
(Less than 100µA on model SMP2-100 and SMP2-115)
(Less than 500µA on model SMP2-230)
G force during first spin = 1250
G force during second spin = 1050
Disconnection device Appliance coupler
Rotor Disposable Capacity 2 or 4
Processing Time approx. 14 minutes
The power outlet on the back of the unit is for future Harvest accessories and should not be used for
anything else. The appliance coupler (also known as the power cord) must always be easily accessible.
2.5 meter power cords supplied by Harvest Technologies, Corp. are the only acceptable means to power
the SmartPReP 2 Centrifuge. Power quality should be that of a typical commercial or hospital environment.
PHYSICAL SPECIFICATIONS
Height: 8.73 in. (22.2 cm) Weight: 34 lb. (15.42 kg)
Width: 16.5 in. (41.91 cm) Weight, Ship: 42 lb. (19.05 kg)
Depth: 18 in. (45.72 cm)
STANDARDS
Harvest reserves the right to discontinue or change specifications without prior notice on all products. The
SmartPReP 2 System has been tested and conforms to the following standards:
UL 61010A-1, First Edition, 2002 - Electrical Equipment for Laboratory Use; Part 1: General Requirements
UL 3101-2-20, First Edition, 1997 - Electrical Equipment for Laboratory Use; Part 2: Laboratory Centrifuges
IEC 60601-1-2:2001 Medical Electrical Equipment - Part 1: General Requirements For Safety 2. Collateral
Standard: Electromagnetic Compatibility - Requirements And Tests
IEC 61326-1:1998 Electrical equipment for measurement, control and laboratory use - EMC requirements -
Part 1: General requirements
CAN/CSA C22.2 No. 1010.1-92 - Safety Requirements for Electrical Equipment for Measurement, Control,
and Laboratory Use, Part 1: General Requirements
CAN/CSA C22.2 No. 1010.2.020-94 - Safety Requirements for Electrical Equipment for Measurement,
Control, and Laboratory Use, Part 2: Particular Requirements for Laboratory Centrifuges
IEC 61010-1:90 / A1: 93 / A2: 95 - Safety Requirements for Electrical Equipment for Measurement, Control,
and Laboratory Use, Part 1: General Requirements
IEC 61010-2-020: 92 - Safety Requirements for Electrical Equipment for Measurement, Control, and
Laboratory Use, Part 2: Particular Requirements for Laboratory Centrifuges
EN 61326 - Electrical Equipment for Measurement, Control and Laboratory Use
EMC Requirements Part 1: General Requirements Includes Amendment A1: 1998;
IEC 61326: 1997 + A1: 1998
ETL Control # 117553
Page 19 of 26 4531200 / 1052
ELECTRICAL SPECIFICATIONS
Model #
SMP2-100
SMP2-115
SMP2-230
Input Voltage (VAC)
100
115
230
Operating Frequency (Hz)
50/60
50/60
50/60
Rated Current (Amps)
5
5
2.5
Fuse rating
5 Amp, time delay,
glass tube, ¼” x 1¼”
5 Amp, time delay,
glass tube, ¼” x 1¼”
2.5 Amp, time delay,
glass tube, 5 x 20mm
ENVIRONMENTAL CONSIDERATIONS
Temperature limit: Operational: 10°C - 30°C (50°F - 86°F)
Storage: -40°C - 66°C (-40°F - 150 °F)
Humidity Limit: Operation: 10-90% non condensing
Storage: 10-90% non condensing
Minimum clearance distance around unit: 3 inches
There is no need to secure the unit with straps or other mounting hardware. Leveling is not required as
long as the unit does not slide off the intended surface.
ELECTROMAGNETIC COMPLIANCE
Special precautions regarding EMC: Do not stack SmartPReP 2 Centrifuges on other medical electrical
equipment. Always check for normal operation when the SmartPReP 2 Centrifuge is moved to a new
location.
Portable and mobile RF communications equipment can effect medical equipment.
WARNING: This equipment/system is intended for use by healthcare professionals only. This
equipment/system may cause radio interference or may disrupt the operation of nearby
equipment. It may be necessary to take mitigation measures, such as reorienting or relocating
the SMP2 or shielding the location.
The use of accessories, transducers and cables other than those supplied by Harvest Technologies, Corp,
may result in increased EMISSIONS or decreased IMMUNITY of the SMP2.
Page 20 of 26 4531200 / 1052
Guidance and manufacturer’s declaration – electromagnetic emissions (Table 201)
The SMP2 is intended for use in the electromagnetic environment specified below. The customer or the user of the SMP2 should assure that it is used in such an
environment.
Emissions test
Compliance
Electromagnetic environment –guidance
RF emissions
CISPR 11
Group 1
The SMP2 uses RF energy only for its internal function. Therefore, its RF emissions are very low and are
not likely to cause any interference in nearby electronic equipment.
RF emissions
CISPR 11
Class A
The SMP2 is suitable for use in all establishments other than domestic and those directly connected to
the public low-voltage power supply network that supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations/flicker emissions
IEC 61000-3-3
Not applicable
Guidance and manufacturer’s declaration – electromagnetic immunity (Table 202)
The SMP2 is intended for use in the electromagnetic environment specified below. The customer or the user of the SMP2 should assure that it is used in such an
environment.
Immunity test
IEC 60601
test level
Compliance level
Electromagnetic environment –
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be wood, concrete or ceramic tile. If floors are covered with
synthetic material, the relative humidity should be at least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
± 2 kV for power
supply lines
± 1 kV for input/output
Lines
± 2 kV for power supply lines
Not applicable for input/output lines,
there are no input/output lines
Mains power quality should be that of a typical commercial or hospital
environment.
Surge
IEC 61000-4-5
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
Mains power quality should be that of a typical commercial or hospital
environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
>95%-0.5 cycles
60%-5 cycles
30%-25 cycles
>95%-250 cycles
Mains power quality should be that of a typical commercial or hospital
environment. If the user of the SMP2 requires continued operation during
power mains interruptions; it is recommended that the SMP2 be powered
from an uninterruptible power supply or a battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
3 A/m @ 50Hz
Power frequency magnetic fields should be at levels characteristic of a
typical location in a typical commercial or hospital environment.
NOTE UT is the arc. mains voltage prior to application of the test level.
Table of contents
Other Terumo Laboratory Equipment manuals
Popular Laboratory Equipment manuals by other brands

Drucker Diagnostics
Drucker Diagnostics Horizon 12 Flex Operator's manual

Kent Scientific
Kent Scientific MouseSTAT Jr Owners manual and set-up guide

KaVo
KaVo EWL 35 operating instructions

Struers
Struers LaboForce-100 instruction manual
Biobase
Biobase BCBL-207 Operation manual

NanoEnTek
NanoEnTek ADAMII-CD34 user manual