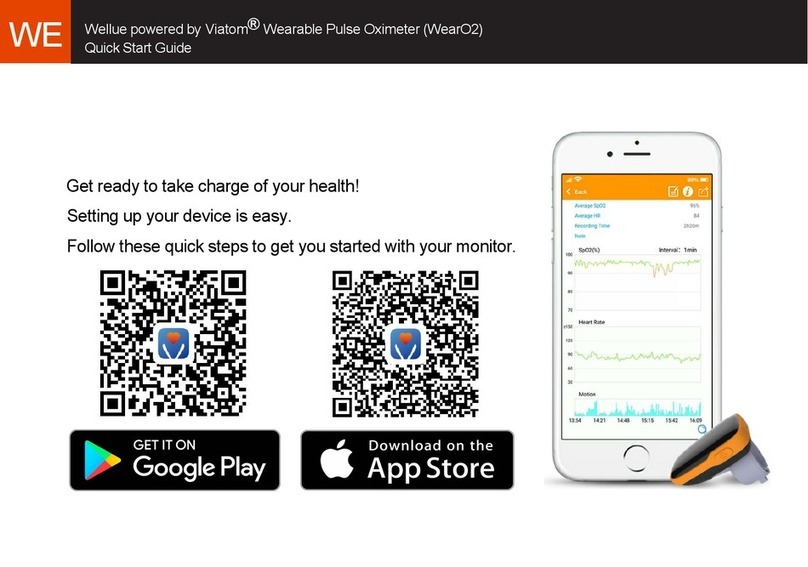
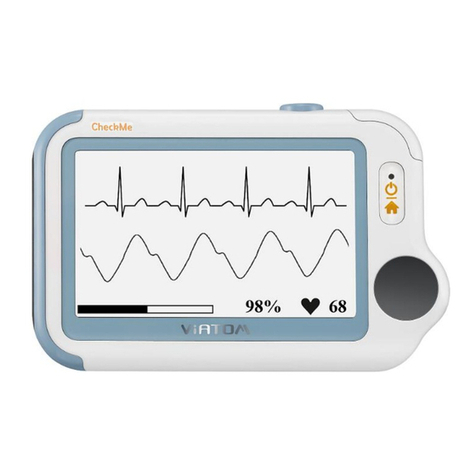
Go to Table of Contents Page 2
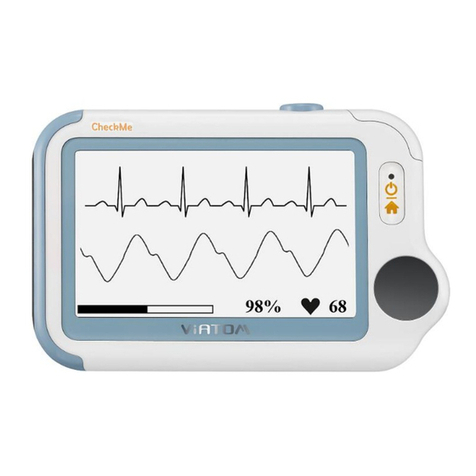
Viatom Checkme™ O2 Wireless Wrist Pulse Oximeter
Distributed by MyCardio LLC for use with the SleepImage System
Issued May 5, 2020
REF D-6.00283 Rev 1
support@sleepimage.com
www.sleepimage.com
Manufactured by Shenzen Viatom Technology Co., Ltd
4E, Building 3, Tingwei Industrial Park
Honglang North 2nd Road, Baoan, 518100
Shenzen, P.R.China www.viatomtech.com
Table of Contents
Introduction ..................................................................................................................................................................3
Included in Box ............................................................................................................................................................3
Using the Recorder with the SleepImage System .......................................................................................................3
Manufacturer Warnings and Cautionary Advices........................................................................................................3
Maintenance .................................................................................................................................................................4
Cleaning the Recorder .................................................................................................................................................4
Charging the Recorder.................................................................................................................................................4
Display .........................................................................................................................................................................4
Power ON/OFF.............................................................................................................................................................4
Pairing the Recorder....................................................................................................................................................4
Performing a SleepImage Study.....................................................................................................................................5
Specifications ................................................................................................................................................................6
Electromagnetic Compatibility ....................................................................................................................................6
EMC Warnings and Cautions......................................................................................................................... 6
Troubleshooting ............................................................................................................................................................6
Type BF-applied part
Manufacturer
CE0197 CE Marking indicating conformance to EC Directive No. 93/42/EEC
MRI Unsafe. Presents hazards in all MR environments as recorder contains strongly ferromagnetic materials
Indicates separate collection for electrical and electronic equipment (WEEE)
Protected against spraying water and against access to hazardous parts with a tool, per IEC 60529
Follow Instructions for Use
Warning and Caution
SN Serial Number
No alarm system
Disclaimer
This document may contain technical inaccuracies or typographical errors. Changes are periodically made to the information herein; these changes will be
incorporated in future revisions of this document. MyCardio does not accept any liability for the use or misuse, direct or indirect, of this product. Users
must accept all responsibility for any results obtained by or concluded from data obtained by the products. The user must accept all responsibility for
results obtained by software from MyCardio. All clinical conclusions and decisions that are made based on the use of this product are the responsibility of
the user. MyCardio does not accept any liability or responsibility for damages arising out of the use of or inability to use this product.