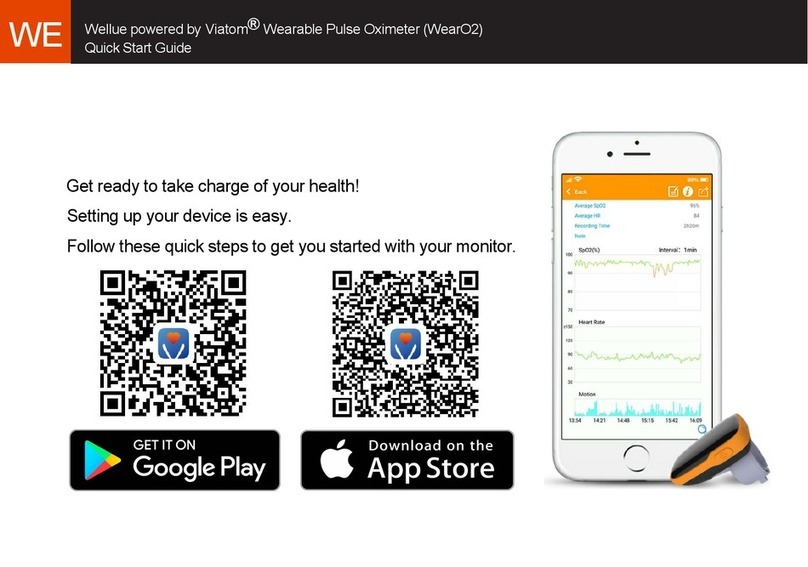
Pulse Oximeter
Instruction for Use
1. Introduction
1.1 Intended use
This Pulse Oximeter is intended to be used for
measuring, displaying and storing of pulse oxygen
saturation (SpO2), pulse rate of adults in home or
healthcare facilities environment.
1.2 Contraindications
No contraindications.
1.3 Warnings and Cautions
DO NOT squeeze the sensor part or apply excessive
force on it.
Do not use this device during MRI examination.
Do not use this device with a defibrillator.
Do not store the device in the following locations:
locations in which the device is exposed to direct
sunlight, high temperatures or levels of moisture, or
heavy contamination; locations near to sources of
water or fire; or locations that are subject to strong
electromagnetic influences.
Do not use the device in a combustible environment
(i.e., oxygen-enriched environment).
Never submerge the device in water or other liquids.
Do not clean the device with acetone or other volatile
solutions.
Do not drop this device or subject it to strong impact.
The device and accessories are provided non-sterile.
Do not place this device in pressure vessels or gas
sterilization device.
Do not dismantle the device, as this could cause
damage or malfunctions or impede the operation of the
device.
Consult your doctor immediately if you experience
symptoms that could indicate acute disease.
Do not self-diagnose or self-medicate on the basis of
this device without consulting your doctor. In particular,
do not start taking any new medication or change the
type and/or dosage of any existing medication without
prior approval.
Use only cables, sensors and other accessories
specified in this manual.
Prolonged continuous monitoring may increase the risk
of undesirable changes in skin characteristics, such as
irritation, reddening, blistering or burns.
Do not open the device cover without authorization.
The cover should only be opened by a qualified service
personnel.
The biocompatibility testing has been performed on the
materials in contact with the person in accordance with
ISO10993.
Do not place the SpO2probe on a finger with edema or
fragile tissue.
Check the SpO2sensor and cable before use. Do not
use a damaged SpO2sensor.
Check the SpO2sensor application site every 6-8 hours
to determine the positioning of the sensor and the
circulation and skin sensitivity of the patient. Patient
sensitivity varies depending on medical status or skin
condition. For patients with poor peripheral blood
circulation or sensitive skin, inspect the sensor site
more frequently.
The functional tester cannot be used to assess the
accuracy of the SpO2sensor or a device.
The device has no alarm system.
Continuous use for a long time may cause allergies,
redness, blistering or burns. Check the wearing
position every 6-8 hours.
The local laws and regulations should be followed
when disposing of the device and accessories.
The battery inside this product cannot be replaced.
1.4 Guide to Symbols
Authorized Representative in the
European Community
Follow Instructions for Use.
MRI unsafe. Presents hazards in
all MR environments as device
contains strongly ferromagnetic
materials.
Protected against spraying water
and against access to hazardous
parts with a tool, per IEC60529.
Atmospheric pressure limitation
Indicate separate collection for
electrical and electronic equipment
(WEEE).
1.5 Unpacking
Device
User Manual
Data/ Charging Cable
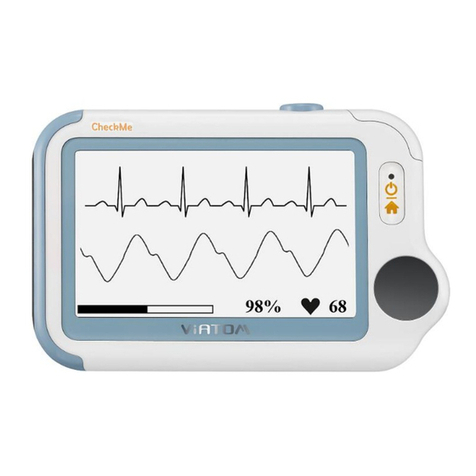
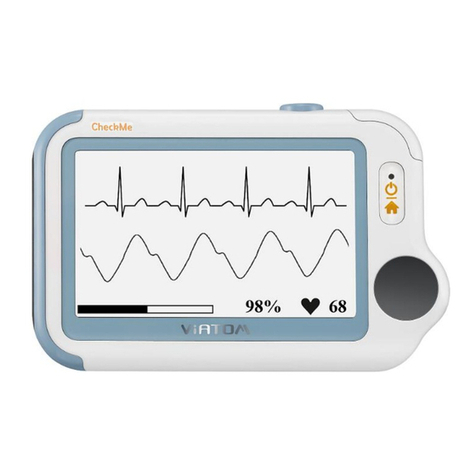
2 Overview
Name: Pulse Oximeter
Model: PO2
1. Touch Switch:
Wake up the screen and switch the interface.
2. Backlit Screen:
Show SpO2value and pulse rate value .
3. USB Charging Port:
Can be charged and data transmission.
4. Sensor:
The device turns on/off automatically in a moment after
you take on/off the sensor.
The blood SpO2and pulse rate can be measured.
3 Using the Device
3.1 Charging
Use the USB cable to charge the product. Connect
the USB cable to a USB charger or to the PC.
There will be a battery logo flash on the device
when charging.
A fully charge will need 2-3 hours. After fully
charged, the device will power off automatically.
Note: The device cannot be used during charging,
and if choosing a third party charging adaptor
(Class II), select one that complies with IEC60950
or IEC60601-1.
3.2 POWER ON/OFF
POWER ON:
Wear the device, it will turn on automatically.
POWER OFF:
The device turns off automatically in a moment
after you take off the sensor.
3.3 Typical steps
1) START. After charge the battery ,wear the
device to power on.
2) STOP. Take off the device, the recording will
be over after the countdown.
3) DATA Uploading. Run your phone to
download data.
3.4 Start working
1) Wear the device on index finger. Try to
move the device along the forefinger to find
out a best fit. Avoid being loose. Loose
wearing causes inaccurate measure.
2) Device will turn on automatically. After a few
seconds, the device will begin to work.
Notice:
If the working time is less than 2 minute, the
data will not be saved.
Please avoid excessive motion.
Please avoid strong ambient light condition.
SpO2measurement principle:
The Pulse Oxiemter is a lightweight, portable
health oximeter for use in the home or in