immediately if you experience symptoms that could indicate acute disease.
Do not self-diagnose or self-medicate on the basis of this device without consulting your
doctor. In particular, do not start taking any new medication or change the type and/or
dosage of any existing medication without prior approval.
This device is not a substitute for a medical examination or your heart or other organ
function, or for medical electrocardiogram recordings, which require more complex
measurements.
It is not possible to use this device to diagnose illness or diseases. This is exclusively the
responsibility of your doctor.
We recommend that you record the ECG curves and other measurements and provide them
to your doctor if required.
Do not use the product in the area of HF surgical equipment, MRI, or CT scanner, or in an
oxygen rich environment.
The battery intended to be changed only by service personnel with the use of a tool, and
replacement by inadequately trained personnel may result in damage or burn.
The patient is an intended operator.
Do not carry out the servicing and maintenance while the product is in use.
The patient can safely use all the functions of the product, and the patient can maintain the
product by carefully reading Chapter 6.
This product emits radio frequencies (RF) in the 2.4 GHz band. DO NOT use this product in
locations where RF is restricted, such as on an aircraft. Turn off the Bluetooth feature in this
product and remove batteries when in RF restricted areas. For further information on
potential restrictions refer to documentation on the Bluetooth usage by the FCC.
DO NOT use this product with other medical electrical (ME) equipment simultaneously. This
may result in incorrect operation of the product and/ or cause an inaccurate blood pressure
readings and/ or EKG recording.
Sources of electromagnetic disturbance may affect this product (e.g. mobile telephones,
microwave cookers, diathermy, lithotripsy, electrocautery, RFID, electromagnetic anti-theft
systems, and metal detectors), please try to stay away from them when making
measurements.
The use of accessories and cables other than those specified or provided by manufacture
could result in increased electromagnetic emission or decreased electromagnetic immunity of
the product and result in improper operation.
Interpretations made by this product are potential findings, not a complete diagnosis of
cardiac conditions. All interpretations should be reviewed by a medical professional for clinical
decision-making.
DO NOT use this product in the presence of flammable anesthetics or drugs.
DO NOT use this product while charging.
Remain still while recording an ECG.
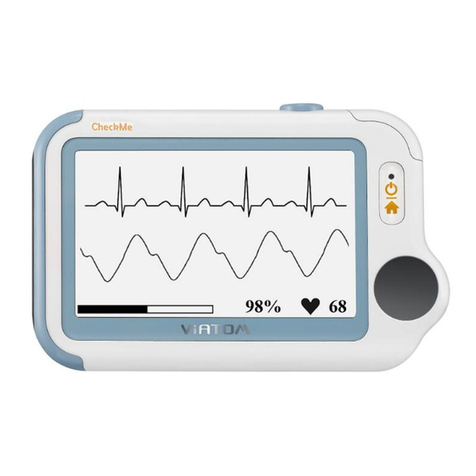
The detectors of ECG have been developed and tested on Lead I and II recordings only.
Do not sterilize this unit in an autoclave or gas sterilizer (EOG, formaldehyde, high density
ozone, etc.)
Do not wash this unit with water.
This product doesn’t provide on-device data backup function.
1. Introduction
1.1 Intended Use
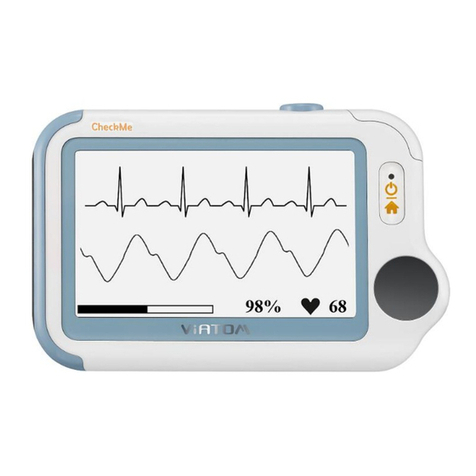
The Portable ECG monitor is intended to be used for measuring, displaying, reviewing and storing
of ECG data and Heart Rate for adult population in home or in healthcare facilities.