2
Contents
Symbols.......................................................................................................................................................................................................... 4
1. Introduction ............................................................................................................................................................................................... 9
2. Electromagnetic compatibility (EMC)................................................................................................................................................... 11
3. Unpacking .................................................................................................................................................................................................12
4. Scope of delivery......................................................................................................................................................................................13
5. Safety notes .............................................................................................................................................................................................14
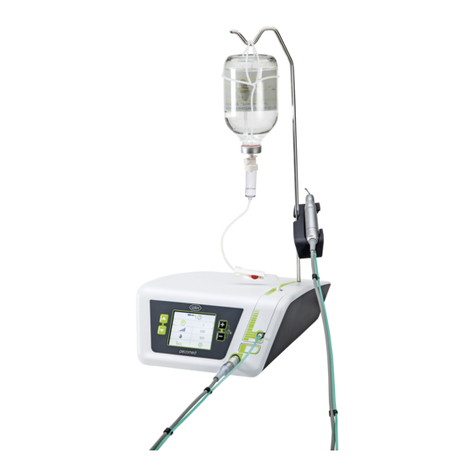
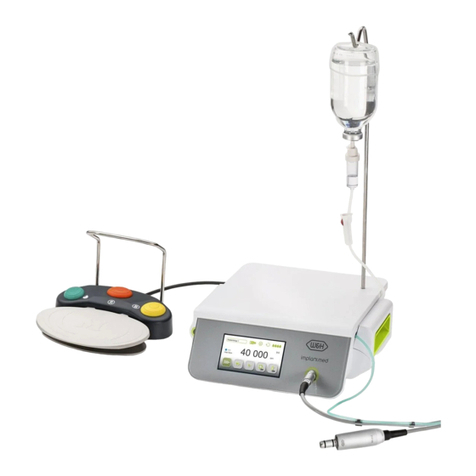
6. Description................................................................................................................................................................................................21
of front panel........................................................................................................................................................................................21
of rear panel........................................................................................................................................................................................22
of foot control S-N2/S-NW .................................................................................................................................................................. 23
of motor with cable ............................................................................................................................................................................. 25
7. Start-up..................................................................................................................................................................................................... 26
8. Control unit .............................................................................................................................................................................................. 27
9. Starting operation ................................................................................................................................................................................... 28
10. Control unit operation........................................................................................................................................................................... 29
Main menu........................................................................................................................................................................................... 29
Menue Navigation ............................................................................................................................................................................... 32
Factory settings.................................................................................................................................................................................. 37
Documentation with USB stick........................................................................................................................................................... 43
ioDent®platform................................................................................................................................................................................. 45
Beacon ................................................................................................................................................................................................ 47
11. Error messages...................................................................................................................................................................................... 48