2
Contents
Symbols.......................................................................................................................................................................................................... 4
1. Introduction ............................................................................................................................................................................................... 7
2. Unpacking .................................................................................................................................................................................................. 9
3. Scope of delivery .................................................................................................................................................................................... 10
4. Safety notes ............................................................................................................................................................................................ 11
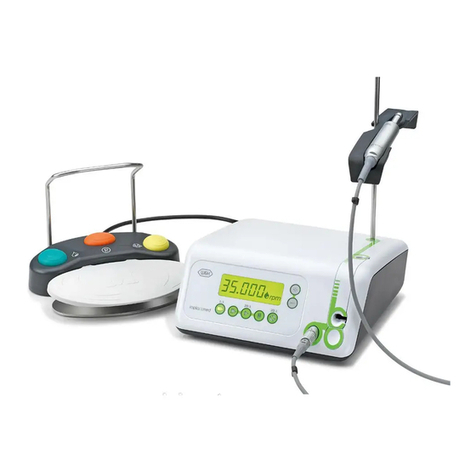
5. Description............................................................................................................................................................................................... 16
6. Start-up .................................................................................................................................................................................................... 19
7. Operation .................................................................................................................................................................................................. 23
8. Icons......................................................................................................................................................................................................... 26
9. Error messages........................................................................................................................................................................................ 29
10. Hygiene and maintenance ................................................................................................................................................................... 31
General notes...................................................................................................................................................................................... 31
Limitations on processing.................................................................................................................................................................. 32
Initial treatment at the point of use .................................................................................................................................................. 33
Manual cleaning.................................................................................................................................................................................. 34
Manual disinfection............................................................................................................................................................................ 35
Automated cleaning and disinfection ............................................................................................................................................... 36
Drying.................................................................................................................................................................................................. 37
Inspection, maintenance and testing ............................................................................................................................................... 38
Packaging............................................................................................................................................................................................ 39
Sterilization......................................................................................................................................................................................... 40
Storage ................................................................................................................................................................................................ 42
11. Service ................................................................................................................................................................................................... 43
12. W&H accessories and spare parts....................................................................................................................................................... 45
13. Technical data........................................................................................................................................................................................ 47