2
Symbols....................................................................................................................................................................................4
1. Introduction .........................................................................................................................................................................7
2. Safety notes ...................................................................................................................................................................... 10
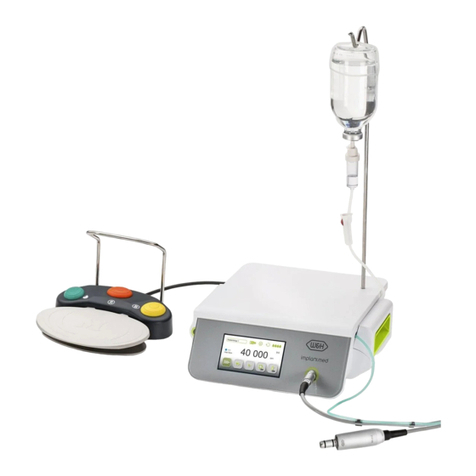
3.Description ......................................................................................................................................................................... 15
4. Instruments ....................................................................................................................................................................... 16
Insert...................................................................................................................................................................................................16
Remove ..............................................................................................................................................................................................17
5. Hygiene and maintenance................................................................................................................................................ 18
General notes..................................................................................................................................................................................... 18
Limitations on processing................................................................................................................................................................ 20
Initial treatment at the point of use..................................................................................................................................................21
Manual cleaning ................................................................................................................................................................................ 22
Automated cleaning and disinfection.............................................................................................................................................. 27
Drying ................................................................................................................................................................................................ 29
Contents