2
Contents
Symbols.......................................................................................................................................................................................................... 4
1. Introduction ............................................................................................................................................................................................... 9
2. Electromagnetic compatibility (EMC)................................................................................................................................................... 11
3. Unpacking .................................................................................................................................................................................................12
4. Scope of delivery......................................................................................................................................................................................13
5. Safety notes .............................................................................................................................................................................................14
6. Description
of front panel........................................................................................................................................................................................21
of rear panel........................................................................................................................................................................................22
of foot control ..................................................................................................................................................................................... 23
of motor with cable ............................................................................................................................................................................. 24
7. Start-up..................................................................................................................................................................................................... 25
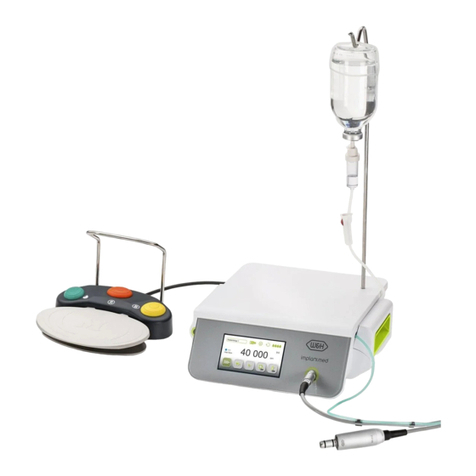
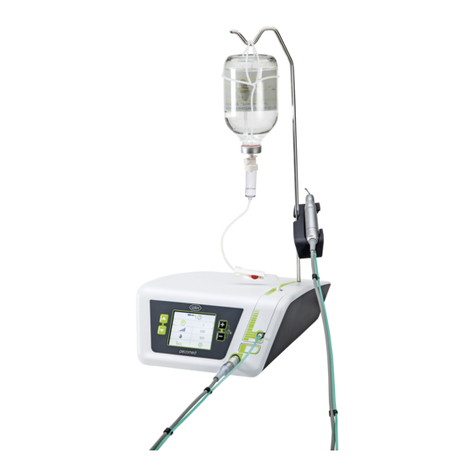
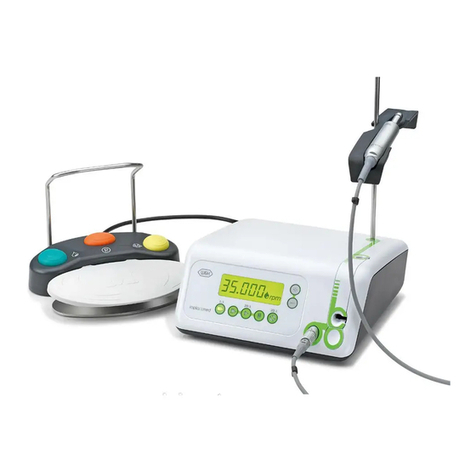
8. Implantmed.............................................................................................................................................................................................. 26
9. Control unit operation
changing program (P1 – P5)............................................................................................................................................................. 27
change speed (P1 – P3) .................................................................................................................................................................... 28
change torque (P4 – P5).................................................................................................................................................................... 29
change coolant volume (P1 – P5)..................................................................................................................................................... 30
10. Operation................................................................................................................................................................................................ 31
11. Restore factory settings....................................................................................................................................................................... 33
12. Thread cutter function (chip breaker mode) ...................................................................................................................................... 36
13. Error messages...................................................................................................................................................................................... 37
14. Hygiene and maintenance
General notes...................................................................................................................................................................................... 38
Limitations on processing.................................................................................................................................................................. 39